 ## Lewis Structures (dot diagrams)
- shows valence $`e^-`$; centre is atomic symbol
- Use family groups to figure out valence $`e^-`$
## Lewis Structures (dot diagrams)
- shows valence $`e^-`$; centre is atomic symbol
- Use family groups to figure out valence $`e^-`$

 ## Trends on the Periodic Table
- `Periodic Table:` Describes **elements** pure susbatances made of only **1** type of Atom.
- The further away the electron is from the nucleus, the more energy it has.
- `Periods:` repeating pattern.
- Metals on **bottom left**, non-metals on **top right**
### Measuring Atomic Radius
- Stack a bunch of them, measure, divide by number of atoms, easy clap :p.
## Trends on the Periodic Table
- `Periodic Table:` Describes **elements** pure susbatances made of only **1** type of Atom.
- The further away the electron is from the nucleus, the more energy it has.
- `Periods:` repeating pattern.
- Metals on **bottom left**, non-metals on **top right**
### Measuring Atomic Radius
- Stack a bunch of them, measure, divide by number of atoms, easy clap :p.
| Trend | You move along a period (row) from left to right | you move down a group (column) from top to bottom |
|---|---|---|
| Number of valence elctrons (electron shells) |
Stays the same | Increases |
| Atomic Radius (size of an atom) |
Decrease due to more protons in the nucleus that attract the electrons, while having the same atomic radius | Increases due to shielding and more energy levels, which actually cancels out and is greater than the force of increasing protons in the nucleus |
| Reactivity of group 1 + 2 metals (i.e How likely are they to lose electrons?) |
Decreases due to smaller atomic radius and more protons in the nucleus | Increases due to larger atomic radius |
| Reactivity of non-metals (Ie. How likely are they to gain electrons?) |
More likely to gain electrons, more protons in nucleus and stronger hold on them | More likely to gain electrons, more protons in nucleus and strong hold on them |
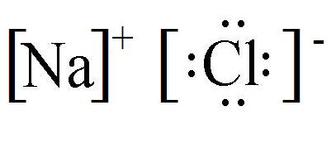 ## Non-Metal Ionic Names
|Element|Name|
|:------|:---|
|Hydrogen|Hydride|
|Boron|Boride|
|Carbon|Carbide|
|Nitrogen|Nitride|
|Oxygen|Oxide|
|Fluorine|Fluoride|
|Silicon|Silicide|
|Phosphide|Phosphide|
|Sulfur|Sulphide/Sulfide|
|Chlorine|Chloride|
|Arsenic|Arsenide|
|Selenium|Selenide|
|Bromine|Bromide|
|Tellurium|Telluride|
|Iodine|Iodide|
|Astatine|Astitide|
## Chemical Nomenclature
- Naming and writing chemical formuals
- According to IUPAC
- Direct relationship beween chemical name and chemical structure
- - Going down diagonally from `aluminium`, we get a pattern of $`3+`$, $`2+`$, $`1+`$ of charge. `Aluminium` has a charge of $`3+`$, `Zinc` has a charge of $`2+`$, and `silver` has a charge of $`1+`$, and they are all mono-valent. (not multi-valent)
- `Galvanize` (rust $`\rightarrow`$ white shield $`\rightarrow`$ cover iron $`\rightarrow`$ prevnet rusting, but I don't think it will be in this unit)
|Formula|Name|
|:------|:---|
|$`NaCl`$|Sodium chloride|
|$`K_3P`$|Potassium phosphide|
|$`Mg_3P_2`$|Magnesium phosphide|
## Polyatomic Ions
- Ions that are made of $`\ge 2`$ atoms.
- Molecules with a charge
- eg. $`CaCo_3`$
- $`Ca \rightarrow`$ Calcium ion $`Ca^{2+}`$ `(Cation)`
- $`CO_3 \rightarrow`$ Carbonate ion $`CO_3^{2-}`$ `(Anion)`
- `Calcium carbonate`
- The ones that are not multi-valent are:
- The first `20` elements
- `alkali metals`
- `alkaline earth metals`
- non-metals (the ones hugging the staircase are also non-metals (some of the `metalloids`))
- `halogens`
- `noble gases`
- If there is more than one polyatomic ion in a formula unit, then surround the ion with brackets/parentheses
- Oxyanion are negative ions with oxygen in them
|Polyatomic Ion Name|Formula (Always Remember The Charge!)|
|:------------------|:------------------------------------|
|Ammonium|$`NH_4^+`$|
|Acetate|$`CH_3COO^-`$|
|Borate|$`BO_3^{3-}`$|
|Chlorate|$`ClO_3^-`$|
|Cyanide|$`CN^-`$|
|Hydroxide|$`OH^-`$|
|Nitrate|$`NO_3^-`$|
|Permanganate|$`MnO_4^-`$|
|Carbonate|$`CO_3^{2-}`$|
|Chromate|$`CrO_4^{2-}`$|
|Dichromate|$`Cr_2O_7^{2-}`$|
|Sulfate|$`SO_4^{2-}`$|
|Phosphate|$`PO_4^{3-}`$|
### Oxyanions
- Nitrate
- Borate
- Carbonate
- Chlorate
- Sulfate
- Phosphate
- And their family members :p.
## Deriving Ions From Parent
|Polyatomic Ion Name|Operation|Chemical Formula|
|:------------------|:--------|:-------|
|**Per**chlor**ate**|(add one extra oxygen to the parent)|$`ClO_4^-`$|
|Chlor**ate**|(**parent**)|**$`ClO_3^-`$**|
|Chlor**ite**|(has one less oxygen than the parent)|$`ClO_2^-`$|
|**Hypo**chlor**ite**|(has two less oxygens than the parent)|$`ClO^-`$|
- Note that the charge remains the same
- Polyatomic ions in the same group on the periodic table form similar polyatomic ions
|**Chlorate**|$`ClO_3^-`$|
|:-----------|:----------|
|Bromate|$`BrO_3^-`$|
## Acidic Oxyanions
- Acids generall have hydrogen ions $`(H^+)`$
- Acidic Oxyanions $`\rightarrow`$ Negatively charged ion with $`O`$ and $`H`$
- Each hydrogen added to a polyatomic ion increases the charge by one, and changes the name:
|Name|Chemical Formula|
|:---|:---------------|
|Hydrogen carbonate ion|$`HCO_3`$|
|Dihydrogen phosphate ion|$`H2PO_4^-`$|
|Monohydrogen phosphate ion|$`HPO_4^{2-}`$|
- For above, we use mono for phosphate to avoid ambigious cases, where $`H_2PO_4^{-}`$ and $`H_2PO_4^{2-}`$ are the same if we don't put `mono` infront. As for the Hyrogen carbonate ion we don't put a mono due to no ambigious cases.
## Molecular Compounds
- Are not made of ions, instead molecules
- Shared pair of electrons -> `covalent bonds`
- `Lone pair` of electrons are electrons that are not shared
- Radicals are atoms with unpaired electrons, very reactive
- Molecules have **no charge**
- Atoms fill their valence shells to form molecules
- Double bond between oxygen atoms in an oxygen molecule
## Properties Of Ionic And Molecular Compounds
|Compound|State at Room Temperature|Solubility In Water|Colour of solution|Conductivity Of Solution|Ionic Or Molecular|
|:-------|:------------------------|:------------------|:-----------------|:-----------------------|:-----------------|
|ammonium chloride|solid|soluble, overtime the substance starts to get smaller and disappears|colourless|conductive|ionic|
|copper $`(II)`$ sulfate|solid|soluable|blue|conductive|ionic|
|sodium chloride|solid|soluble|colourless|conductive|ionic|
|calcium hydroxide|solid|slightly soluable|white|slightly conductive|ionic|
|sodium hydroxide|solid|soluble|colourless|conductive|ionic|
|sucrose|solid|soluble|colourless|not conductive|molecular|
|iodine|solid|not soluble|yellow|not conductive|molecular|
|hydrochloric acid|aqueous|soluble|colourless|conductive|molecular|
|ethanol|liquid|soluble|colourless|nont conductive|molecular|
|nitrogen gas|gas|N/A|N/A|N/A|molecular|
|carbon dioxide (dissolved in water)|gas|slightly soluble|colourless|a tiny bit conductive|molecular|
## Generalizations
|Classification of substances|Phase at room temperature|Solubility in water|Colour of solution|Conductivity of solution|
|:---------------------------|:------------------------|:------------------|:-----------------|:-----------------------|
|Ionic|Solid|Soluble|colourless, white|Conductive|
|Molecualr|liquid, gas, or solid|non-soluble|Has distinct colour?|Not really conductive|
## Binary Molecular Compounds
- 2 different kinds of atom in molecule
- Eg. $`CO_2 \rightarrow`$ Carbon Diox**ide** $`\rightarrow`$ 2nd atom has `ide`.
- $`CO \rightarrow`$ Carbon Monox**ide** $`\rightarrow`$ If 1st atom is mono, drop `mono`
### Greek Prefix For Number Of Atom
|Prefix|Name|Preifx|Name|
|:-----|:---|:-----|:---|
|1|mono|6|hexa|
|2|di|7|hepta|
|3|tri|8|octa|
|4|tetra|9|nona|
|5|penta|10|deca|
- `Diatomic Molecules` The **gens**, Hydrogen, Nitrogen, Oxygen, Halogen
### Common Names
- $`NH_3 \rightarrow`$ Ammonia
- $`H_2O \rightarrow`$ Water
- $`CH_4 \rightarrow`$ Methane
### Elements found As Molecules In Nature
- $`H_{2(g)}, Cl_{2(g)}, Br_{2(g)}, I_2, N_2, O_2, F_2`$
|Chemical Formula|Lewis Structure|What does the molecular model look like?|Name|
|:---------------|:-------------:|:--------------------------------------:|:---|
|$`H_2`$|
## Non-Metal Ionic Names
|Element|Name|
|:------|:---|
|Hydrogen|Hydride|
|Boron|Boride|
|Carbon|Carbide|
|Nitrogen|Nitride|
|Oxygen|Oxide|
|Fluorine|Fluoride|
|Silicon|Silicide|
|Phosphide|Phosphide|
|Sulfur|Sulphide/Sulfide|
|Chlorine|Chloride|
|Arsenic|Arsenide|
|Selenium|Selenide|
|Bromine|Bromide|
|Tellurium|Telluride|
|Iodine|Iodide|
|Astatine|Astitide|
## Chemical Nomenclature
- Naming and writing chemical formuals
- According to IUPAC
- Direct relationship beween chemical name and chemical structure
- - Going down diagonally from `aluminium`, we get a pattern of $`3+`$, $`2+`$, $`1+`$ of charge. `Aluminium` has a charge of $`3+`$, `Zinc` has a charge of $`2+`$, and `silver` has a charge of $`1+`$, and they are all mono-valent. (not multi-valent)
- `Galvanize` (rust $`\rightarrow`$ white shield $`\rightarrow`$ cover iron $`\rightarrow`$ prevnet rusting, but I don't think it will be in this unit)
|Formula|Name|
|:------|:---|
|$`NaCl`$|Sodium chloride|
|$`K_3P`$|Potassium phosphide|
|$`Mg_3P_2`$|Magnesium phosphide|
## Polyatomic Ions
- Ions that are made of $`\ge 2`$ atoms.
- Molecules with a charge
- eg. $`CaCo_3`$
- $`Ca \rightarrow`$ Calcium ion $`Ca^{2+}`$ `(Cation)`
- $`CO_3 \rightarrow`$ Carbonate ion $`CO_3^{2-}`$ `(Anion)`
- `Calcium carbonate`
- The ones that are not multi-valent are:
- The first `20` elements
- `alkali metals`
- `alkaline earth metals`
- non-metals (the ones hugging the staircase are also non-metals (some of the `metalloids`))
- `halogens`
- `noble gases`
- If there is more than one polyatomic ion in a formula unit, then surround the ion with brackets/parentheses
- Oxyanion are negative ions with oxygen in them
|Polyatomic Ion Name|Formula (Always Remember The Charge!)|
|:------------------|:------------------------------------|
|Ammonium|$`NH_4^+`$|
|Acetate|$`CH_3COO^-`$|
|Borate|$`BO_3^{3-}`$|
|Chlorate|$`ClO_3^-`$|
|Cyanide|$`CN^-`$|
|Hydroxide|$`OH^-`$|
|Nitrate|$`NO_3^-`$|
|Permanganate|$`MnO_4^-`$|
|Carbonate|$`CO_3^{2-}`$|
|Chromate|$`CrO_4^{2-}`$|
|Dichromate|$`Cr_2O_7^{2-}`$|
|Sulfate|$`SO_4^{2-}`$|
|Phosphate|$`PO_4^{3-}`$|
### Oxyanions
- Nitrate
- Borate
- Carbonate
- Chlorate
- Sulfate
- Phosphate
- And their family members :p.
## Deriving Ions From Parent
|Polyatomic Ion Name|Operation|Chemical Formula|
|:------------------|:--------|:-------|
|**Per**chlor**ate**|(add one extra oxygen to the parent)|$`ClO_4^-`$|
|Chlor**ate**|(**parent**)|**$`ClO_3^-`$**|
|Chlor**ite**|(has one less oxygen than the parent)|$`ClO_2^-`$|
|**Hypo**chlor**ite**|(has two less oxygens than the parent)|$`ClO^-`$|
- Note that the charge remains the same
- Polyatomic ions in the same group on the periodic table form similar polyatomic ions
|**Chlorate**|$`ClO_3^-`$|
|:-----------|:----------|
|Bromate|$`BrO_3^-`$|
## Acidic Oxyanions
- Acids generall have hydrogen ions $`(H^+)`$
- Acidic Oxyanions $`\rightarrow`$ Negatively charged ion with $`O`$ and $`H`$
- Each hydrogen added to a polyatomic ion increases the charge by one, and changes the name:
|Name|Chemical Formula|
|:---|:---------------|
|Hydrogen carbonate ion|$`HCO_3`$|
|Dihydrogen phosphate ion|$`H2PO_4^-`$|
|Monohydrogen phosphate ion|$`HPO_4^{2-}`$|
- For above, we use mono for phosphate to avoid ambigious cases, where $`H_2PO_4^{-}`$ and $`H_2PO_4^{2-}`$ are the same if we don't put `mono` infront. As for the Hyrogen carbonate ion we don't put a mono due to no ambigious cases.
## Molecular Compounds
- Are not made of ions, instead molecules
- Shared pair of electrons -> `covalent bonds`
- `Lone pair` of electrons are electrons that are not shared
- Radicals are atoms with unpaired electrons, very reactive
- Molecules have **no charge**
- Atoms fill their valence shells to form molecules
- Double bond between oxygen atoms in an oxygen molecule
## Properties Of Ionic And Molecular Compounds
|Compound|State at Room Temperature|Solubility In Water|Colour of solution|Conductivity Of Solution|Ionic Or Molecular|
|:-------|:------------------------|:------------------|:-----------------|:-----------------------|:-----------------|
|ammonium chloride|solid|soluble, overtime the substance starts to get smaller and disappears|colourless|conductive|ionic|
|copper $`(II)`$ sulfate|solid|soluable|blue|conductive|ionic|
|sodium chloride|solid|soluble|colourless|conductive|ionic|
|calcium hydroxide|solid|slightly soluable|white|slightly conductive|ionic|
|sodium hydroxide|solid|soluble|colourless|conductive|ionic|
|sucrose|solid|soluble|colourless|not conductive|molecular|
|iodine|solid|not soluble|yellow|not conductive|molecular|
|hydrochloric acid|aqueous|soluble|colourless|conductive|molecular|
|ethanol|liquid|soluble|colourless|nont conductive|molecular|
|nitrogen gas|gas|N/A|N/A|N/A|molecular|
|carbon dioxide (dissolved in water)|gas|slightly soluble|colourless|a tiny bit conductive|molecular|
## Generalizations
|Classification of substances|Phase at room temperature|Solubility in water|Colour of solution|Conductivity of solution|
|:---------------------------|:------------------------|:------------------|:-----------------|:-----------------------|
|Ionic|Solid|Soluble|colourless, white|Conductive|
|Molecualr|liquid, gas, or solid|non-soluble|Has distinct colour?|Not really conductive|
## Binary Molecular Compounds
- 2 different kinds of atom in molecule
- Eg. $`CO_2 \rightarrow`$ Carbon Diox**ide** $`\rightarrow`$ 2nd atom has `ide`.
- $`CO \rightarrow`$ Carbon Monox**ide** $`\rightarrow`$ If 1st atom is mono, drop `mono`
### Greek Prefix For Number Of Atom
|Prefix|Name|Preifx|Name|
|:-----|:---|:-----|:---|
|1|mono|6|hexa|
|2|di|7|hepta|
|3|tri|8|octa|
|4|tetra|9|nona|
|5|penta|10|deca|
- `Diatomic Molecules` The **gens**, Hydrogen, Nitrogen, Oxygen, Halogen
### Common Names
- $`NH_3 \rightarrow`$ Ammonia
- $`H_2O \rightarrow`$ Water
- $`CH_4 \rightarrow`$ Methane
### Elements found As Molecules In Nature
- $`H_{2(g)}, Cl_{2(g)}, Br_{2(g)}, I_2, N_2, O_2, F_2`$
|Chemical Formula|Lewis Structure|What does the molecular model look like?|Name|
|:---------------|:-------------:|:--------------------------------------:|:---|
|$`H_2`$|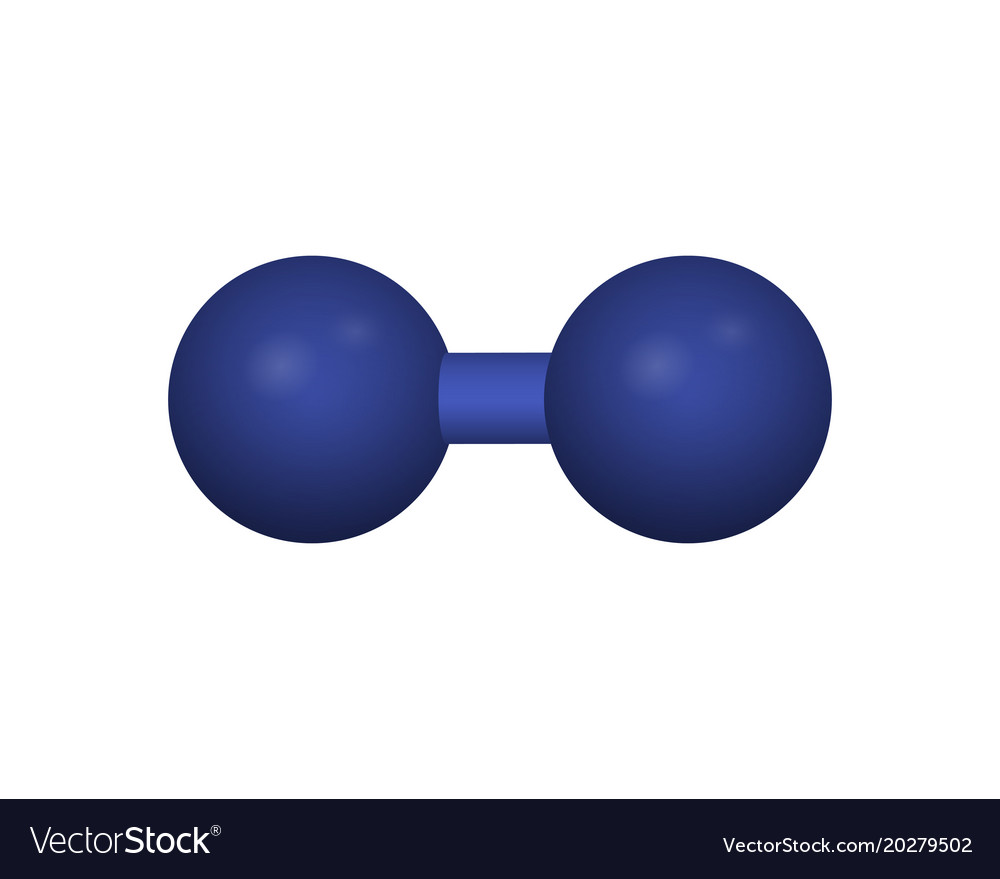 |Hydrogen|
|$`O_2`$|
|Hydrogen|
|$`O_2`$|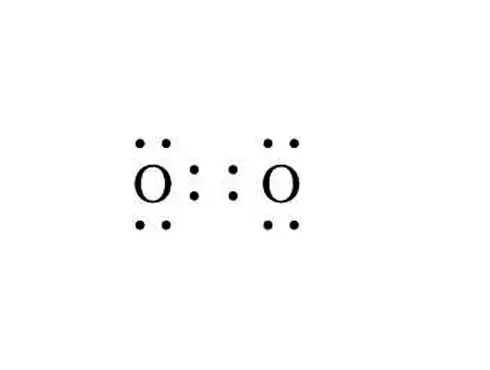 |
| |Oxygen|
|$`N_2`$|
|Oxygen|
|$`N_2`$| |
| |Nitrogen|
|$`I_2`$|
|Nitrogen|
|$`I_2`$| |
| |Iodine|
|$`H_2O`$|
|Iodine|
|$`H_2O`$| |
| |Water|
|$`NH_3`$|
|Water|
|$`NH_3`$| |
| |Ammonia|
|$`CO_2`$|
|Ammonia|
|$`CO_2`$|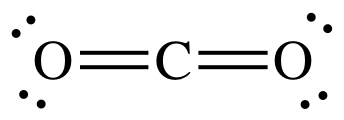 |
| |Carbon dioxide|
|$`SBr_2`$|
|Carbon dioxide|
|$`SBr_2`$| |
|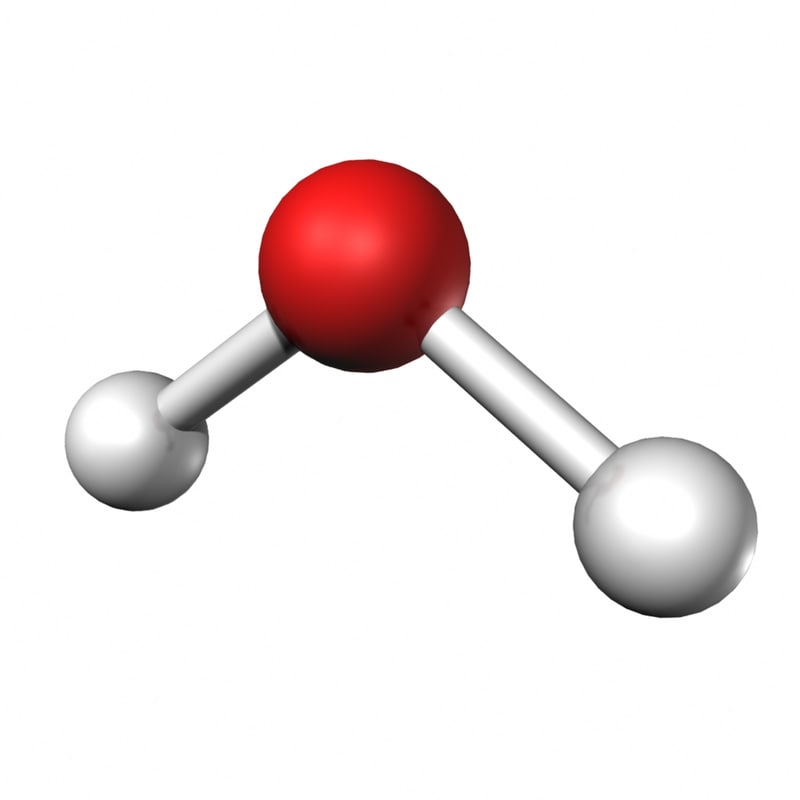 |Sulfur dibromide|
|$`O_3`$|
|Sulfur dibromide|
|$`O_3`$| |
| |Ozone|
|$`CF_4`$|
|Ozone|
|$`CF_4`$|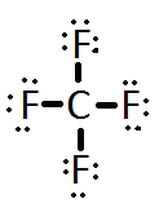 |
|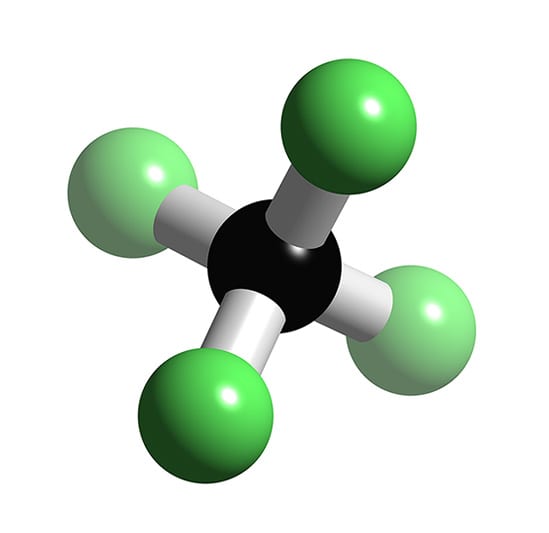 |Carbon tetrafluoride|
|$`SiH_4`$|
|Carbon tetrafluoride|
|$`SiH_4`$| |
| |Silicon tetrahydride|
|$`OH^-`$|
|Silicon tetrahydride|
|$`OH^-`$|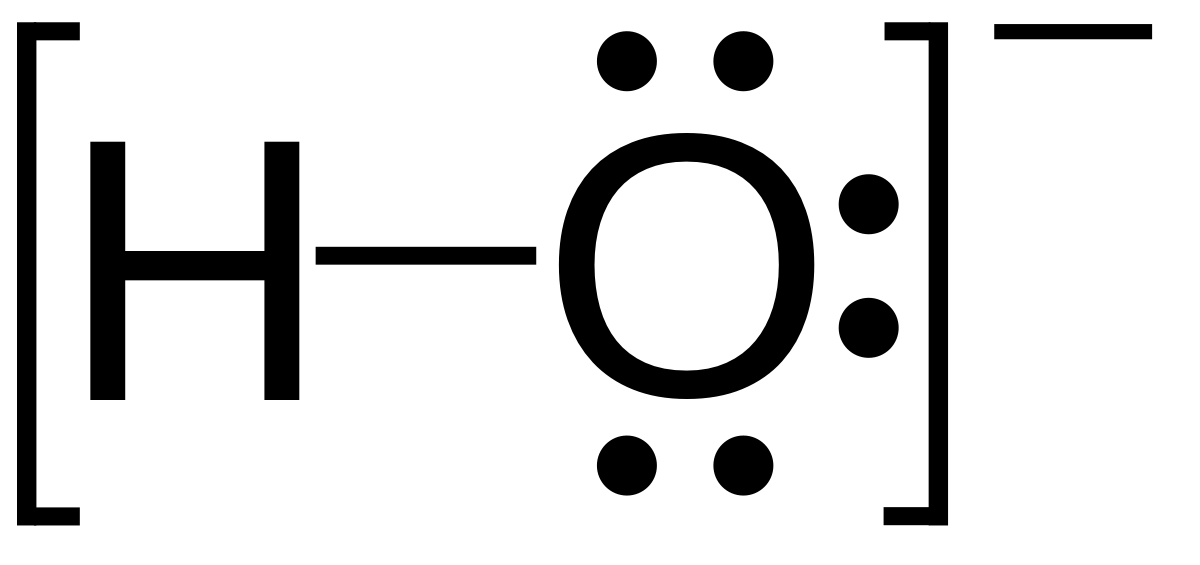 |
|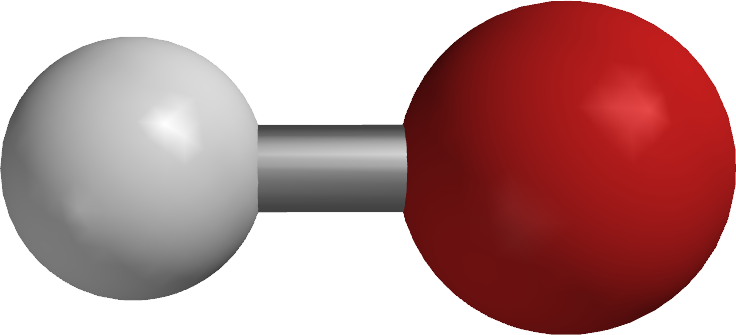 |Hydroxide ion|
|$`H_3O^+`$|
|Hydroxide ion|
|$`H_3O^+`$| |Hydrodium ion|
|Dots representing shared pairs of elections|Lines representing shared pairs of electrons|
|:-----------------------------------------:|:------------------------------------------:|
|
|Hydrodium ion|
|Dots representing shared pairs of elections|Lines representing shared pairs of electrons|
|:-----------------------------------------:|:------------------------------------------:|
| |
| |
|