mirror of
https://gitlab.com/magicalsoup/Highschool.git
synced 2025-03-14 00:51:46 -04:00
16 KiB
16 KiB
Unit 1: Chemistry
Matter: has mass, takes up space.- fundamental unit -> ATOMS
- One
formula unit- repeating strucure in an ionic compound that has the simplest ratio of ions in the compound- Can be represented in Chemical Formula (e.g )
- Ions are particles with charges
Models: Allows people to make accuratepredictionsaboutbehaviorof MATTER.Atom: Smallest unit of element that still retains its properties. Made of subatomic particlesAtomic mass unit:
Atoms
- Atoms are the smallest unit of an element that still retains its properties
- Atoms are made of subatomic particles
- Relative Charge: compared to something.
- AMU = atomic mass units
- atmoic notation
- an isotope is an atom (or atoms) of an element with a unique # of neutrons
| Name | Symbol | Relative mass (amu) | Location | Relative Charge |
|---|---|---|---|---|
| Protons | nucleus | |||
| Neutrons | nucleus | |||
| Electrons | in orbit around nucleus (shell, energy level) |
IUPAC
| Letter | Definition |
|---|---|
| I | International |
| U | Union |
| P | Pure and |
| A | Applied |
| C | Chemistry |
- Involved in studying, varifying information (eg the periodic table -> Describes the elements -> pure susbtances made of only one kind of atom),and publishing.
- Standarize the information for the public
Bohr Rutherford
- Electrons in uncharged atom, # protons # electrons
- Mass of an atom is the weighted average if akk usitioes if element
Atomic Notation, Top number is the mass, bottom number is the atomic number.

Lewis Structures (dot diagrams)
- shows valence ; centre is atomic symbol
- Use family groups to figure out valence


Trends on the Periodic Table
Periodic Table:Describes elements pure susbatances made of only 1 type of Atom.- The further away the electron is from the nucleus, the more energy it has.
Periods:repeating pattern.- Metals on bottom left, non-metals on top right
Measuring Atomic Radius
- Stack a bunch of them, measure, divide by number of atoms, easy clap :p.
| Trend | You move along a period (row) from left to right | you move down a group (column) from top to bottom |
|---|---|---|
|
Number of valence electrons and electron shells |
Valence shells stays the same, while electrons increases | Valence shells increases, while electrons stays the same |
|
Atomic Radius (size of an atom) |
Decrease due to more protons in the nucleus that attract the electrons, while having the same atomic radius | Increases due to shielding and more energy levels, which actually cancels out and is greater than the force of increasing protons in the nucleus |
|
Reactivity of group 1 + 2 metals (i.e How likely are they to lose electrons?) |
Decreases due to smaller atomic radius and more protons in the nucleus | Increases due to larger atomic radius |
|
Reactivity of non-metals (Ie. How likely are they to gain electrons?) |
More likely to gain electrons, more protons in nucleus and stronger hold on them | More likely to gain electrons, more protons in nucleus and strong hold on them |
Rows
- Same energy level in each period
- Same number of valence electrons in each group
- Across a row/period more in nucleus greater attraction to
- Atomic radius decreases as you move acroos a row/period, due to more protons in the nucleus that attract the negatives.
- Atomic radius is the from the center of the atom (nucleus) to the outer most shell (valence shell)
Columns
- down a column, increase of energy level, as you move down
- every atom has only one valence shell (cause its the most outer shell)
- if valence shell is further away from the nucleus, less attractive force between nucleus and valence
- more energy levels where can be
- Negative electrons are repeling the valence shell electrons
(shielding) Shielding“inner electrons” repel valence electrons and “block” attraction force between valence electrons and nucleus- Atomic radius increases as you move down a column/group
Metals
- They tend to lose electrons
- They are shiny, ductile, malleable, conductive
- They have a weak/loose hold on electrons
- Most metals are considered to be multi-metals
(
multi-valent)- can form ions of differing charges
- add roman numerals to the ions name to indicate its charge, for example, iron() oxide.
Metalloids: non-metals with same metallic or metals with non-metalic properies`
Non-Metals
- They are dull, bad conductors - insulators
- Tend to gain electrons
- The have a strong hold on electrons
- Usually non-ductile nor malleable
Bonds
- An ionic bond is a bond between a negative ion and a positive ion (so a anion and a cation)
- An convalent bond is a bond between 2 non-metals
- An ion is a charged particle
- An anion is formed when an particle gains electrons
- An cation is formed when an particle loses electrons
- We can use modesl(e.g Lewis dot diagrams) to show bonding
- Atoms will lose or gain electrons to achieve noble gas configuration The most common stable ion. (eg, if loses electrons, it becomes like , if gains an electron, it becomes like )
- To show that atoms are different than ions, we put square brackets around it , then we put superscript on the top right to show its charge, (if the charge is only a , we just put a instead of )
- Example of ionic bond:
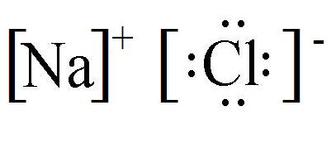
Non-Metal Ionic Names
| Element | Name |
|---|---|
| Hydrogen | Hydride |
| Boron | Boride |
| Carbon | Carbide |
| Nitrogen | Nitride |
| Oxygen | Oxide |
| Fluorine | Fluoride |
| Silicon | Silicide |
| Phosphide | Phosphide |
| Sulfur | Sulphide/Sulfide |
| Chlorine | Chloride |
| Arsenic | Arsenide |
| Selenium | Selenide |
| Bromine | Bromide |
| Tellurium | Telluride |
| Iodine | Iodide |
| Astatine | Astitide |
Chemical Nomenclature
- Naming and writing chemical formuals
- According to IUPAC
- Direct relationship beween chemical name and chemical structure
- Going down diagonally from
aluminium, we get a pattern of , , of charge.Aluminiumhas a charge of ,Zinchas a charge of , andsilverhas a charge of , and they are all mono-valent. (not multi-valent)Galvanize(rust white shield cover iron prevnet rusting, but I don’t think it will be in this unit)
- Going down diagonally from
| Formula | Name |
|---|---|
| Sodium chloride | |
| Potassium phosphide | |
| Magnesium phosphide |
Polyatomic Ions
- Ions that are made of atoms.
- Molecules with a charge
- eg.
- Calcium ion
(Cation) - Carbonate
ion
(Anion) Calcium carbonate
- Calcium ion
- The ones that are not multi-valent are:
- The first
20elements alkali metalsalkaline earth metals- non-metals (the ones hugging the staircase are also non-metals (some
of the
metalloids)) halogensnoble gases
- The first
- If there is more than one polyatomic ion in a formula unit, then surround the ion with brackets/parentheses
- Oxyanion are negative ions with oxygen in them
| Polyatomic Ion Name | Formula (Always Remember The Charge!) |
|---|---|
| Ammonium | |
| Acetate | |
| Borate | |
| Chlorate | |
| Cyanide | |
| Hydroxide | |
| Nitrate | |
| Permanganate | |
| Carbonate | |
| Chromate | |
| Dichromate | |
| Sulfate | |
| Phosphate |
Oxyanions
- Nitrate
- Borate
- Carbonate
- Chlorate
- Sulfate
- Phosphate
- And their family members :p.
Deriving Ions From Parent
| Polyatomic Ion Name | Operation | Chemical Formula |
|---|---|---|
| Perchlorate | (add one extra oxygen to the parent) | |
| Chlorate | (parent) | |
| Chlorite | (has one less oxygen than the parent) | |
| Hypochlorite | (has two less oxygens than the parent) |
- Note that the charge remains the same
- Polyatomic ions in the same group on the periodic table form similar polyatomic ions
| Chlorate | |
|---|---|
| Bromate |
Acidic Oxyanions
- Acids generall have hydrogen ions
- Acidic Oxyanions Negatively charged ion with and
- Each hydrogen added to a polyatomic ion increases the charge by one, and changes the name:
| Name | Chemical Formula |
|---|---|
| Hydrogen carbonate ion | |
| Dihydrogen phosphate ion | |
| Monohydrogen phosphate ion | |
| Hydrogen Sulfate | |
| Hydrogen Carbonate |
- For above, we use mono for phosphate to avoid ambigious cases, where
and are the same if we don’t
put
monoinfront. As for the Hyrogen carbonate ion we don’t put a mono due to no ambigious cases.
Molecular Compounds
- Are not made of ions, instead molecules
- Shared pair of electrons ->
covalent bonds Lone pairof electrons are electrons that are not shared- Radicals are atoms with unpaired electrons, very reactive
- Molecules have no charge
- Atoms fill their valence shells to form molecules
- Double bond between oxygen atoms in an oxygen molecule
Properties Of Ionic And Molecular Compounds
| Compound | State at Room Temperature | Solubility In Water | Colour of solution | Conductivity Of Solution | Ionic Or Molecular |
|---|---|---|---|---|---|
| ammonium chloride | solid | soluble, overtime the substance starts to get smaller and disappears | colourless | conductive | ionic |
| copper sulfate | solid | soluable | blue | conductive | ionic |
| sodium chloride | solid | soluble | colourless | conductive | ionic |
| calcium hydroxide | solid | slightly soluable | white | slightly conductive | ionic |
| sodium hydroxide | solid | soluble | colourless | conductive | ionic |
| sucrose | solid | soluble | colourless | not conductive | molecular |
| iodine | solid | not soluble | yellow | not conductive | molecular |
| hydrochloric acid | aqueous | soluble | colourless | conductive | molecular |
| ethanol | liquid | soluble | colourless | nont conductive | molecular |
| nitrogen gas | gas | N/A | N/A | N/A | molecular |
| carbon dioxide (dissolved in water) | gas | slightly soluble | colourless | a tiny bit conductive | molecular |
Generalizations
| Classification of substances | Phase at room temperature | Solubility in water | Colour of solution | Conductivity of solution |
|---|---|---|---|---|
| Ionic | Solid | Soluble | colourless, white | Conductive |
| Molecualr | liquid, gas, or solid | non-soluble | Has distinct colour? | Not really conductive |
Binary Molecular Compounds
- 2 different kinds of atom in molecule
- Eg. Carbon
Dioxide 2nd atom has
ide. - Carbon
Monoxide If 1st atom is mono, drop
mono
- Eg. Carbon
Dioxide 2nd atom has
Greek Prefix For Number Of Atom
| Prefix | Name | Preifx | Name |
|---|---|---|---|
| 1 | mono | 6 | hexa |
| 2 | di | 7 | hepta |
| 3 | tri | 8 | octa |
| 4 | tetra | 9 | nona |
| 5 | penta | 10 | deca |
Diatomic MoleculesThe gens, Hydrogen, Nitrogen, Oxygen, Halogen
Common Names
- Ammonia
- Water
- Methane
Elements found As Molecules In Nature
| Chemical Formula | Lewis Structure | What does the molecular model look like? | Name |
|---|---|---|---|
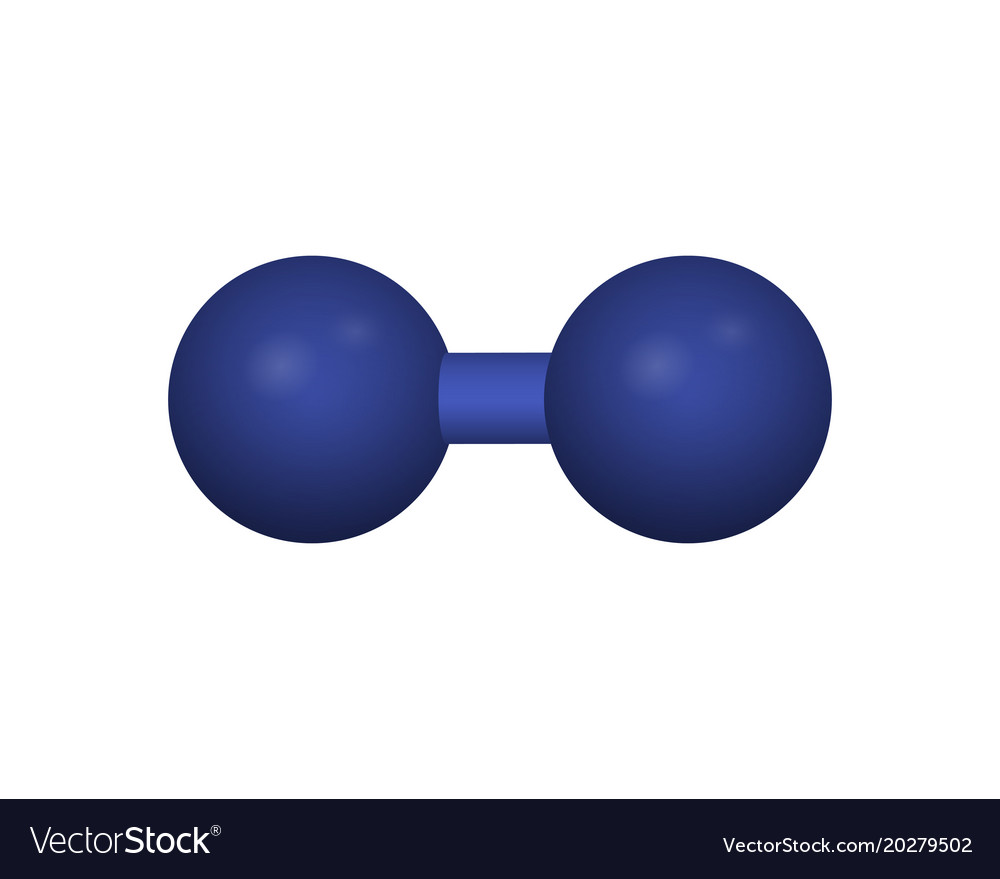 |
Hydrogen | ||
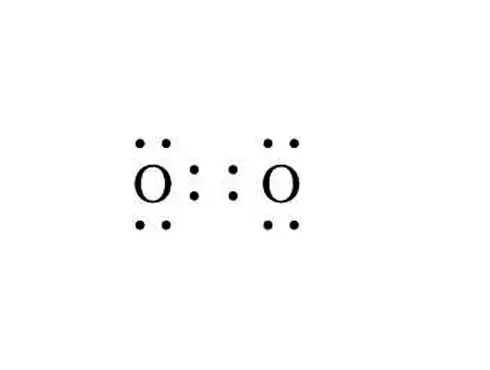 |
 |
Oxygen | |
 |
 |
Nitrogen | |
 |
 |
Iodine | |
 |
 |
Water | |
 |
 |
Ammonia | |
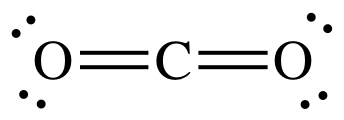 |
 |
Carbon dioxide | |
 |
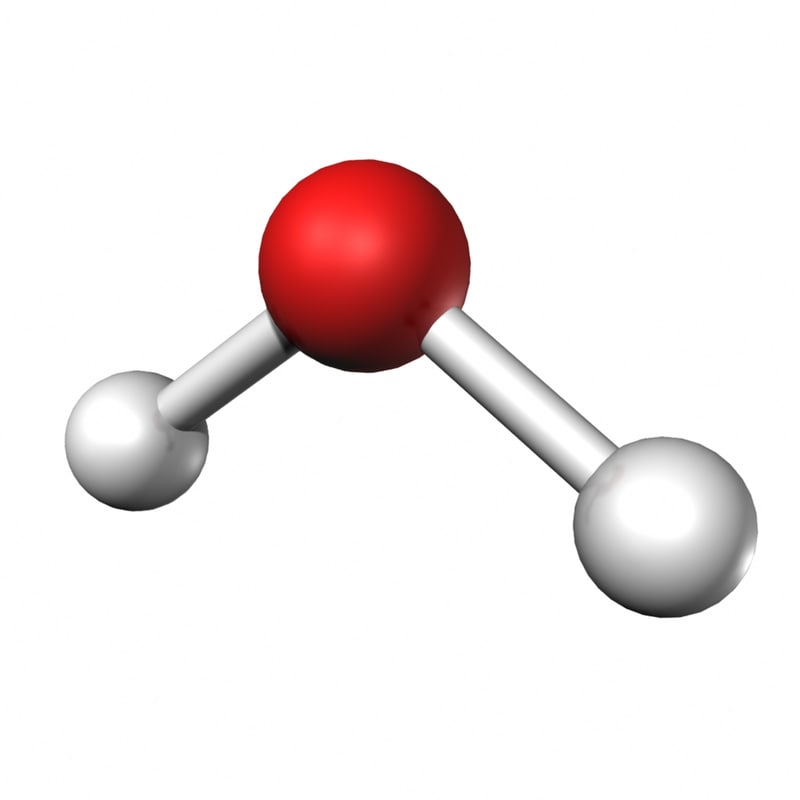 |
Sulfur dibromide | |
 |
 |
Ozone | |
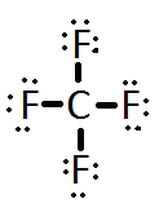 |
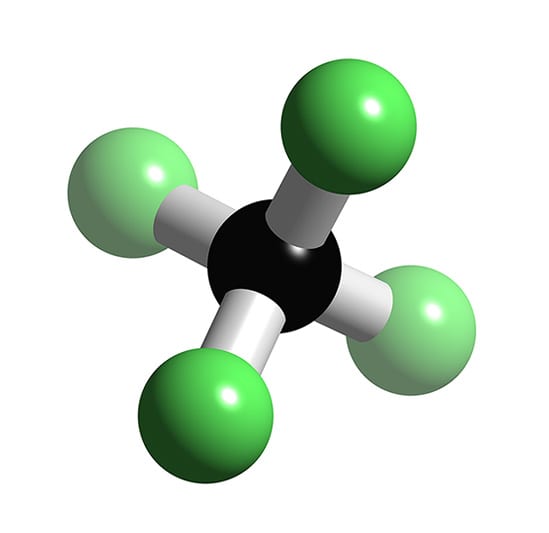 |
Carbon tetrafluoride | |
 |
 |
Silicon tetrahydride | |
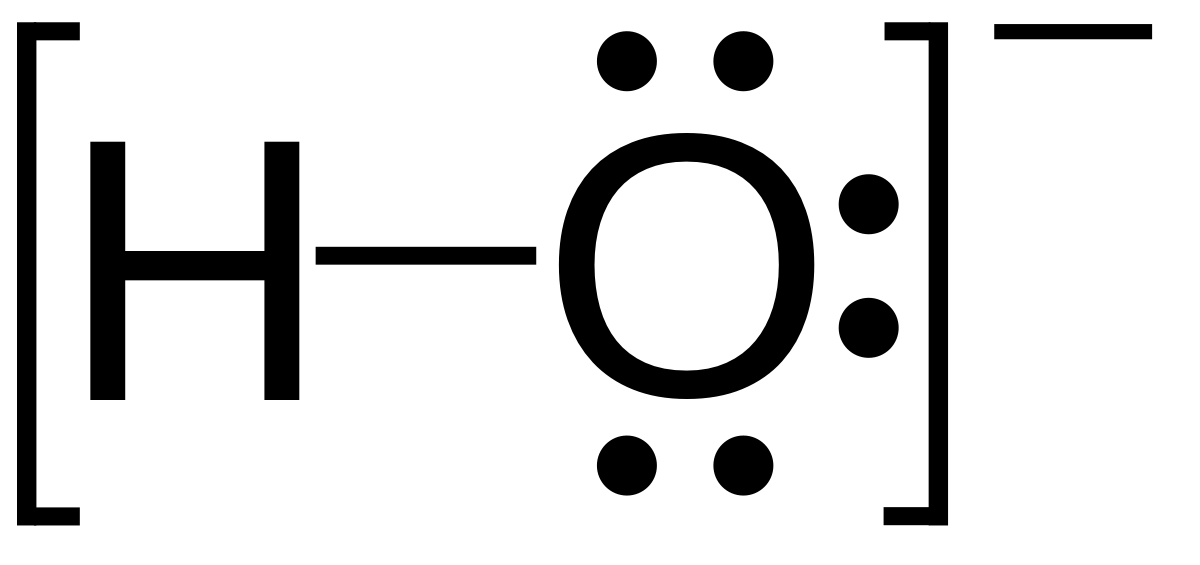 |
 |
Hydroxide ion | |
 |
Hydrodium ion |
| Dots representing shared pairs of elections | Lines representing shared pairs of electrons |
|---|---|
 |
 |