19 KiB
Unit 1
Unit 2
Chemistry Vocabulary List
| Word | Definition (or diagram/translation) |
|---|---|
| Particle Theory of Matter | Theory that describes the composition and behaviour of matter as being composed of small particles with empty space |
| Matter | Substance that has mass and occupies space |
| Mechanical Mixture | A heterogeneous mixture which one can physically separate |
| Suspension | A heterogeneous mixture where insoluble solid particles are distributed throughout a fluid, floating freely/td> |
| Alloy | A combination of 2+ metals |
| Mixture | A substance that is made up of at least 2 types of particles |
| Qualitative property | A property of a substance that is not measured and doesn’t have a numerical value, such as colour, odour, and texture |
| Qualitative observation | An numerical observation |
| Precipitate | A solid that separates from a solution |
| Density | A measure of how much mass is contained in a given unit volume of a substance; calculated by dividing the mass of a sample of its volume (mass/volume) |
| Element | Element An element is made up of the same atoms throughout, and cannot be broken down further |
| Metal | a solid material that is typically hard, shiny, malleable, fusible, and ductile, with good electrical and thermal conductivity |
| Pure substance | A substance that is made up of only one type of particle |
| Atom | The smallest unit of matter found in substances |
| Solution | A uniform mixture of 2 or more substances |
| Colloid | is substance with small particles suspended in it, unable to be separated by gravity |
| Emulsion | A mixture of 2 insoluble liquids, in which one liquid is suspended in the other |
| Physical Property | Characteristic of a substance that can be determined without changing the makeup of the substance |
| Characteristic | A physical property that is unique to a substance and can be used to identify the substance |
| Periodic Table | a table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure (and hence similar chemical properties) appear in vertical columns. |
| Compound | Compounds are chemically joined atoms of different elements |
| Non-Metal | A substance that isn’t a metal |
Physical Properties
- A characeristic of a substance that can be determined without changing the composition (“make-up”) of that substance
- Characteristics can be determinded using your 5 senses and measuring
instruments
- smell, taste, touch, hearing, sight
- scales, tape, measuring meter
Qualitative and Quantitative Properties
| Type | Definition | Example |
|---|---|---|
| Quantitative Property | A property that IS measured and has
a numerical value |
Ex.
Temperature, height, mass, density |
| Qualitative Property | A property that is NOT measured and has
no numerical value |
Ex.
Colour, odor, texture |
Quantitative physical Properties
Density: amount ofstuff(or mass) per unit volume (g/cm3)Freezing Point: point where water solidifies (0oC)Melting Point: point where water liquefies (0oC)Boiling Point: point where liquid phase becomes gaseous (100oC)
Common Qualitative Physical Properties
| Type | Definition | Example |
|---|---|---|
| Lustre | Shininess of dullness Referred to as high or low lustre depending on the shininess |
|
| Clarity | The ability to allow light through | Transparent (Glass)
Translucent (Frosted Glass) Opaque
(Brick) |
| Brittleness | Breakability or flexibility Glass would be considered as brittle whereas slime/clay are flexible |
|
| Viscosity | The ability of a liquid or gas to resist
flow or not pour readily through Refer to as more or less viscous |
Molasses is more viscous, water is less (gases tend to get”thicker as heated; liquids get runnier) |
| Hardness | The relative ability to scratch or be
scratched by another substance Referred to as high or low level of hardness |
Can use a scale (1 is wax, 10 is diamond) |
| Malleability | the ability of a substance
to be hammered into a thinner sheet or molded |
Silver is malleable Play dough/pizza dough is less glass is not malleable |
| Ductility | the ability of a substance to be pulled into a finer strand | Pieces of copper can be drawn into thin wires, ductile |
| Electrical Conductivity | The ability of a substance to allow
electric current to pass through it Refer to as high and low conductivity |
Copper wires have high conductivity Plastic has no conductivity |
| Form: Crystalline Solid | Have their particles arranged in an orderly geometric pattern | Salt and Diamonods |
| Form: Amorphous Solid | Have their particles randomly distributed without any long-range-pattern | Plastic, Glass, Charcoal |
Chemical Property
A characteristic (property) of a substance that describes its ability to undergo
changes to its composition to produce one of more new substances. AKA BEHAVIOUR. Everything has one!Cannot be determined by physical propertiesE.g. ability of nails /cars to rust
Fireworks are explosive
Denim is resistant to soap, but is combustible
Baking soda reacts with vinegar and cake ingredients to rise
Bacterial cultures convert milk to cheese, grapes to wine, cocoa to chocolate
CLR used to clean kettles, showerheads because it breaks down minerals
Silver cleaner for tarnished jewellery, dishes because silver reacts with air to turn black
Elements
- At the present time
118elements are known. - These elements vary widely in their abundance
- For example, only five elements account for over 90% of the Earth’s crust: oxygen, silicon, aluminum, iron and calcium.
Naming of Ionic Bonds
- Write cation (metal) first
- Write anion (non-metal) second
- Change the ending of the non-metal to
ide.
Decomposition
- A chemical change used to break compounds down into simpler substances
- Energy must be ADDED
- Using electricity
- Adding thermal energy
Catalyst
- Substance that accelerates a chemical change without being consumed OR changed itself
Uses of Hydrogen Peroxide
- On cuts/scraps
- Blood has a catalyst = see bubbling O2
- Cleans contact lenses
- Bubbling removes dirt
- Bleaches
- React with compounds that provide color
- RESULT = no colour (bleach blond hair/teeth)
Unit 3: Biology
The Sphere’s of Earth
Atmosphere
- The layer of
gasesabove Earth’s surface, extending upward for hundreds of kilometers.78% nitrogen gas.21% oxygen gas.< 1% argon, water vapour, carbon dioxide & other gases.
- Critical to (almost all) life on Earth.
- Acts like a blanket & moderates surface temperature.
- Insulation prevents excessive heating during the day & excessive cooling during the night.
- Average surface temperature droup from 15C to -18C.
- Blocks some solar radiation (most ultraviolet light).
Biosphere
- The regions of Earth where
living organismsexist. - Describes the locations in which life can exist within the lithosphere, atmosphere and hydrosphere.
- Biosphere is thin in comparison to diameter of the Earth.
- ALL conditions required for life must be met and maintained within this thin layer of ground, water, and nutrients to survive.
Hydrosphere
- All the
waterfound on Earth, above and below the Earth’s surface. - Includes
- Oceans
- Lakes
- Ice
- Ground Water
- Clouds
- 97% of water on Earth is in the oceans.
Lithosphere
- The
hard partof Earth’s surface. - Rocky outer shell of Earth.
- Consists of:
- Rocks and minerals that make up mountains, ocean floors, and Earth’s solid landscape -Thickness: 50 - 150km.
Terms
Biotic: Living components (their remains AND features)- Bears, insects, micro-organisms, nests
Abiotic: Non-living components- Physical/chemical components
- Temperature, wind, humidity, precipitation, minerals, air pressure
Sustainability: The ability to maintain natural ecological conditions without interruption, weakening, or loss of value.Population- All of the individuals of a single species in a particular area
Community- Individual from all of the DIFFERENT populations (communities of different species)
Ecosystem- Term given to the community and its interactions with the abiotic environment
Sustainable Ecosystem- An ecosystem that is maintained through natural processes
- Ecological niche:
- Every species interacts with other species and with its environment in a unique way. This is its role in an ecosystem (e.g. what it eats, what eats it, how it behaves, etc.)
Types of Energy
Radiant Energy
- Energy that travels through EMPTY SPACE
Thermal Energy
- Form of energy TRANSFERED DURING HEATING/COOLING
- Keeps the Earth’s surface warm
- CANNOT provide organisms with energy to grow & function ## Successions
Succession: The gradual and usually predictable changes in the composition of a community and the abiotic condtions following a disturbance.Primary
- on newly epxposed ground, such asa following a volcanic eruption.
Secondary
- in a partially distrubed ecosystem, such as following a forest fire.
- Human caused disturbances. #### Light Energy
- VISIBLE forms of radiant energy
- Can be used by some organisms (CANNOT be stored)
Chemical Energy
- Used by living organisms to perform functions (growth, reproduction, etc.)
- MUST be replaced as it is used
It starts with the sun …
- Energy radiates from the sun (UV)
- Earth is hit with the UV or light energy
- 70% of radiant energy is absorbed by
Hydrosphere&Lithosphere - Converted into thermal energy
- Warms the atmosphere, evaporates water & produces winds
- What happens with the rest?
- Approx.
30%is reflected back into space 0.023%absorbed by living organisms through photosynthesis
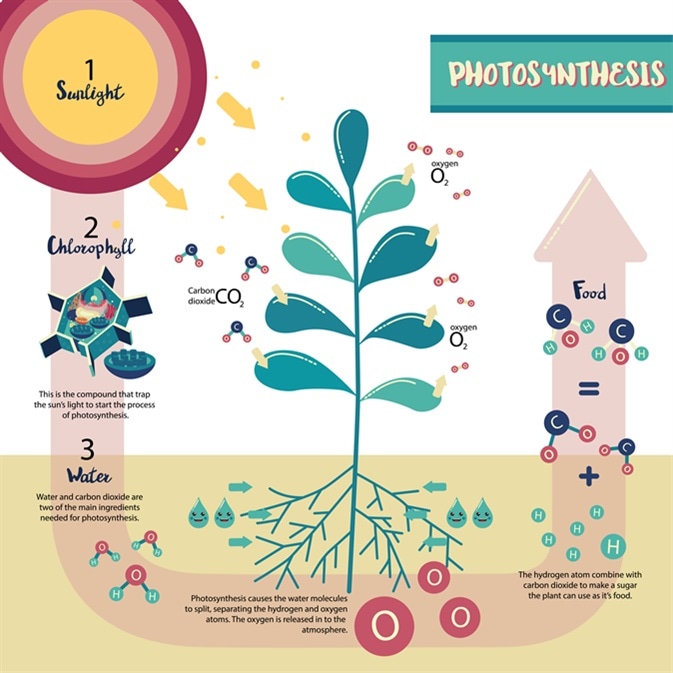
PHOTOSYNTHESIS
PHOTO- Light
SYNTHESIS- Put together
- The process in which the Sun’s energy (LIGHT) is converted (put together with) into chemical energy AS GLUCOSE (sugar)
PHOTOSYNTHESIS
- In order for photosynthesis to happen the plant will NEED:
- IGHT
- CARBON DIOXIDE
- WATER
- CHLOROPHYLL (found inside the cell of a plant)
Photosynthesis
Light energy turns the water & carbon dioxide into oxygen and glucose (sugar)
Sugar formed contains stored chemical energy
Stored in:
- Roots
- Stems
- Leaves
- Seeds
Plants convert the sugar to starch (for storage)
SOME sugars are rearranged to form:
- Carbohydrates (oxygen, hydrogen, carbon)
- Proteins (oxygen, hydrogen, carbon and NITROGEN)
Why is this important?
Animals cannot make their own food (glucose, energy)
- Must get our food from plants.
Plants are the first step in the food chain
Oxygen released during photosynthesis is necessary for all living things
PRODUCER: Organism that makes its own energy-rich food using the Sun’s energy
- GREEN PLANTS
- Green comes from chlorophyll (captures light)
CONSUMER: Organism that obtains its energy from consuming other organisms
Cellular Respiration
Process of converting sugar into carbon dioxide, water and energy
Makes stored energy available for use
Takes place in the mitochondria
Steps in Cellular Respiration
- Mitochondria takes in nutrients
- Glucose and Oxygen
- Breaks both nutrients down
- Creates energy for the cell
REVERSE of Photosynthesis
- Sugar breaks down into CARBON DIOXIDE and
WATER
- Release of energy when this happens
- Sugar breaks down into CARBON DIOXIDE and
WATER
Cellular Respiration
INFO
- Original energy stored in the sugar is released
- Occurs continuously
- Does NOT require light energy
BOTH producers AND consumers perform cellular respiration
ALL humans are consumers (unless you’re the hulk)
Feeding Relationship
Energy flow through an ecosystem in one direction, from the sun or inorganic compounds to autotrophs (producers) and then to various hetrotrophs (consumers).
Food are a series of steps in which organisms transfers energy by eating or eaten (pg. 43).
Food webs show the complex interactions within an ecosystem (pg. 44).
Each step in a food chain or web is called a
trophiclevel. Producers make up the first step, consumers make up the higher levels. E.g. first trophic level are producers, second trophic level are primary consumers, etc.
ECOLOGICAL PYRAMIDS
Food chains and food webs do not give any information about the numbers of organisms involved.
This information can be shown through ecological pyramids.
An ecological pyramid is a diagram that shows the amount of energy or matter contained within each trophic level in a food web or food chain.
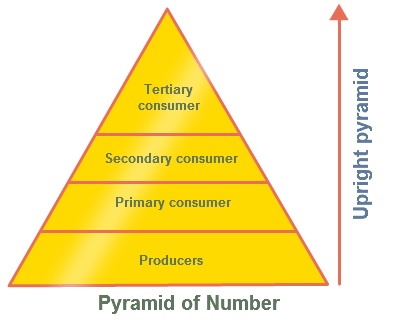
| Pyramid | Description | Picture |
|---|---|---|
| Pyramid of Biomass | Show the total amout of
living tissue available at each trophic level.
This shows the amount of tissue available for the next
trophic level. Biomass is preferred to the use of numbers of organisms because individual organisms can vary in size. It is the total mass (not the size) that is
important. Sometimes it’s inverted. Pyramid of biomass records the total dry organic matter of organisms at each trophic level in a given area of an ecosystem. |
 |
| Numbers Pyramids | Shows the number of organisms at each
trophic level per unit area of an ecosystem. Because each trophic level harvests only about one tenth of the energy
from the level below, it can support only about one 10th
the amount of living tissue. Can be inverted: 1 large tree supports
thousands of organisms living on it Pyramid of numbers displays the number of individuals |
 |
| Energy Pyramid | Shows the amount of energy input to each
trophic level in a given area of an ecosystem over an extended
period. CANNOT be inverted, due to energy transfers Only 10% of the energy available within one trophic level is transferred to organisms at the next trophic level |
 |
NOTE FOR ENERGY PYRAMIDS: In nature, ecological
efficiency varies from 5% to 20% energy
available between successive trophic levels (95% to
80% loss). About 10% efficiency is a general rule.
Rule of 10’s at each level.
Cycles
| Cycle | Description | Picture |
|---|---|---|
| Water Cycle | Continuous movement of water on, above and below the surface of the Earth |  |
| Carbon Cycle | Main Pathway – in and out of living matter |  |
| Nitrogen Cycle | Main Pathway - In and out of plants and is helped by bacteria |  |
Water Cycle
Key Terms:
- Water moves from one reservoir to another (ocean to atmosphere,
river to lake)
- Evaporation, Condensation, Precipitation, Percolation (Infiltration), Run-off
- Forms: Solid (ice), Liquid (water), Gas (vapour)
STEPS/PROCESS:
- Exchange of energy leads to:
- Temperature Change, Climate
- Condenses 🡪 occurs during cooler temp
- Evaporation 🡪 happens during warmer temp
- Evaporation:
- purifies the water
- New fresh water for the land
- Flow of liquid water and ice
- Transports minerals across the globe
- Reshaping the geological features of Earth
- Erosion and sedimentation
Carbon Cycle
- Fourth most abundant element in universe
- Building block of all living things
STEPS/PROCESSES
- All living organisms contain carbon
- CO2 is a waste product of cellular respiration
- Plants use carbon dioxide and water to form simple sugars (photosynthesis)
- Light Energy –> Chemical Energy
Nitrogen Cycle
- The most abudant gas in the atmopshere (~78%)
Nitrogen Fixation: The process that causes the strong two-atom nitrogen molecules found in the atmopshere to break apart so they can combine with other atoms.Nitrogen gets fixed: Whenit is combined with oxygen or hydrogen.- An essential component of DNA, RNA, and protenis - the building blocks of life.
- Atmopspheric nitrogen = N2
- Most living organisms are
unableto use this form of nitrogen - Therefore, must be converted to a usable form! ### STEPS/PROCESSES
- Most living organisms are
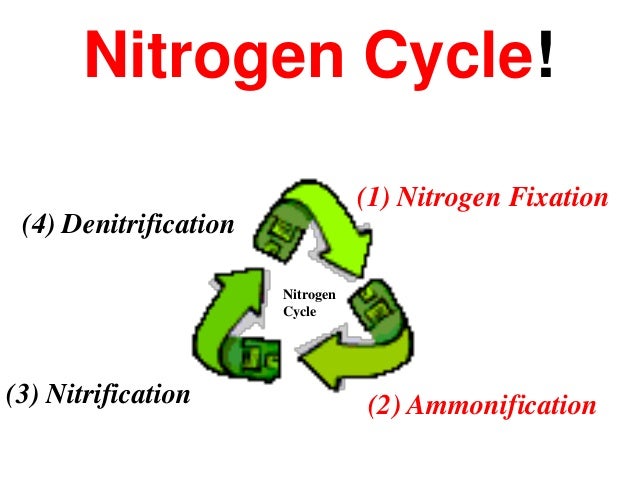
Nitrogen Fixation
- Most of the nitrogen used by living things is taken from the
atmosphere by certain bacteria in a process called
nitrogen fixation. - These microorganisms convert nitrogen gas into a variety of nitrogen containing compounds such as nitrates, nitrites, and ammonia
- Lightning and UV radiation also fix small amounts of it
- Humans add nitrogen to soil through fertilizer
- 3 ways nitrogen to get fixed
- Atmopheric Fixation
- Industrial Fixation
- Biological Fixation
- 2 types
- Free living Bacteria
- Highly specialized bacteria live in the soil and have the ability to combine atmospheric nitrogen with hydrogen to make ammonium(NH4+).
- Nitrogen changes into ammonium.
- Symbiotic Relationship Bacteria
- Bacteria live in the roots of legume family plants and provide the plants with ammonium(NH4+) in exchange for the plant’s carbon and a protected biome.
- Free living Bacteria
- 2 types
Benefits of Succession
- Provides a mechanism by which ecosysmtems maintain their long term sustainability.
- Allows ecosystems to recover from natural or human caused distrubances.
- Offers hope (New Orleans, New Jersey, Florida, Puerto Rica).
- Time needed is very long.
- Original cause o disturbance must be eliminated.
- Not all disturbances can be repaired.
- Disturbances can be repaired through humans actions that support the natural processes of succession.
Biodiversity
- The variety