mirror of
https://gitlab.com/magicalsoup/Highschool.git
synced 2025-01-23 16:11:46 -05:00
36 KiB
36 KiB
Unit 1: Science skills
Unit 2: Chemistry
Chemistry Vocabulary List
| Word | Definition (or diagram/translation) |
|---|---|
| Particle Theory of Matter | Theory that describes the composition and behaviour of matter as being composed of small particles with empty space |
| Matter | Substance that has mass and occupies space |
| Mechanical Mixture | A heterogeneous mixture which one can physically separate |
| Suspension | A heterogeneous mixture where insoluble solid particles are distributed throughout a fluid, floating freely/td> |
| Alloy | A combination of 2+ metals |
| Mixture | A substance that is made up of at least 2 types of particles |
| Qualitative property | A property of a substance that is not measured and doesn’t have a numerical value, such as colour, odour, and texture |
| Qualitative observation | An numerical observation |
| Precipitate | A solid that separates from a solution |
| Density | A measure of how much mass is contained in a given unit volume of a substance; calculated by dividing the mass of a sample of its volume (mass/volume) |
| Element | Element An element is made up of the same atoms throughout, and cannot be broken down further |
| Metal | a solid material that is typically hard, shiny, malleable, fusible, and ductile, with good electrical and thermal conductivity |
| Pure substance | A substance that is made up of only one type of particle |
| Atom | The smallest unit of matter found in substances |
| Solution | A uniform mixture of 2 or more substances |
| Colloid | is substance with small particles suspended in it, unable to be separated by gravity |
| Emulsion | A mixture of 2 insoluble liquids, in which one liquid is suspended in the other |
| Physical Property | Characteristic of a substance that can be determined without changing the makeup of the substance |
| Characteristic | A physical property that is unique to a substance and can be used to identify the substance |
| Periodic Table | a table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure (and hence similar chemical properties) appear in vertical columns. |
| Compound | Compounds are chemically joined atoms of different elements |
| Non-Metal | A substance that isn’t a metal |
| Physical Change |
A change in which the composition of the substance remains
unalteredandno new substances are produced
|
| Chemical Change |
A change in the starting substance and the production of ONE
or more new substances Original substance does not disappear BUT the composition is rearranged |
| Molecule | Two or more non-metal atoms joined together |
| Diatomic Molecules |
Molecules that only consists of 2 elements H O F BR I N CL - hyrodgen,
oxygen, fluorine, bromine,
iodine, nitrogen, chlorine.
|
| Ions | A Charged particle, that results from a loss (cation - positve, less electrons) or gain (anion - negative, more electrons) of electrons when bonding |
| Electron | Negatively Charged |
| Proton | Positively Charged |
| Neutron | Neutral Charged |
| Ionic Charge | The sum of the positive and negative charges in a ion |
| Covalent Bond | The sharing of electrons between atoms when bonding |
| Valence Electrons | Number of electrons on the most outer orbit/shell of the element |
Particle Theory of Matter
- Matter is made up of tiny particles.
- Particles of Matter are in constant motion.
- Particles of Matter are held together by very strong electrical forces.
- There are empty spaces between the particles of matter that are very large compared to the particles themselves.
- Each substance has unique particles that are different from the particles of other substances.
Physical Properties
- A characeristic of a substance that can be determined without changing the composition (“make-up”) of that substance
- Characteristics can be determinded using your 5 senses and measuring
instruments
- smell, taste, touch, hearing, sight
- scales, tape, measuring meter
Qualitative and Quantitative Properties
| Type | Definition | Example |
|---|---|---|
| Quantitative Property | A property that IS measured and has
a numerical value |
Ex.
Temperature, height, mass, density |
| Qualitative Property | A property that is NOT measured and has
no numerical value |
Ex.
Colour, odor, texture |
Density

Quantitative physical Properties
Density: amount ofstuff(or mass) per unit volume (g/cm3)Freezing Point: point where water solidifies (0oC)Melting Point: point where water liquefies (0oC)Boiling Point: point where liquid phase becomes gaseous (100oC)
Common Qualitative Physical Properties
| Type | Definition | Example |
|---|---|---|
| Lustre | Shininess of dullness Referred to as high or low lustre depending on the shininess |
|
| Clarity | The ability to allow light through | Transparent (Glass)
Translucent (Frosted Glass) Opaque
(Brick) |
| Brittleness | Breakability or flexibility Glass would be considered as brittle whereas slime/clay are flexible |
|
| Viscosity | The ability of a liquid or gas to resist
flow or not pour readily through Refer to as more or less viscous |
Molasses is more viscous, water is less (gases tend to get”thicker as heated; liquids get runnier) |
| Hardness | The relative ability to scratch or be
scratched by another substance Referred to as high or low level of hardness |
Can use a scale (1 is wax, 10 is diamond) |
| Malleability | the ability of a substance
to be hammered into a thinner sheet or molded |
Silver is malleable Play dough/pizza dough is less glass is not malleable |
| Ductility | the ability of a substance to be pulled into a finer strand | Pieces of copper can be drawn into thin wires, ductile |
| Electrical Conductivity | The ability of a substance to allow
electric current to pass through it Refer to as high and low conductivity |
Copper wires have high conductivity Plastic has no conductivity |
| Form: Crystalline Solid | Have their particles arranged in an orderly geometric pattern | Salt and Diamonods |
| Form: Amorphous Solid | Have their particles randomly distributed without any long-range-pattern | Plastic, Glass, Charcoal |
Chemical Property
- A characteristic (property) of a substance that describes its
ability to undergo
changes to its composition to produce one of more new substances. AKA BEHAVIOUR. Everything has one! Cannot be determined by physical properties- E.g. ability of nails /cars to rust
- Fireworks are explosive
- Denim is resistant to soap, but is combustible
- Baking soda reacts with vinegar and cake ingredients to rise
- Bacterial cultures convert milk to cheese, grapes to wine, cocoa to chocolate
- CLR used to clean kettles, showerheads because it breaks down minerals
- Silver cleaner for tarnished jewellery, dishes because silver reacts with air to turn black.
Periodic Table
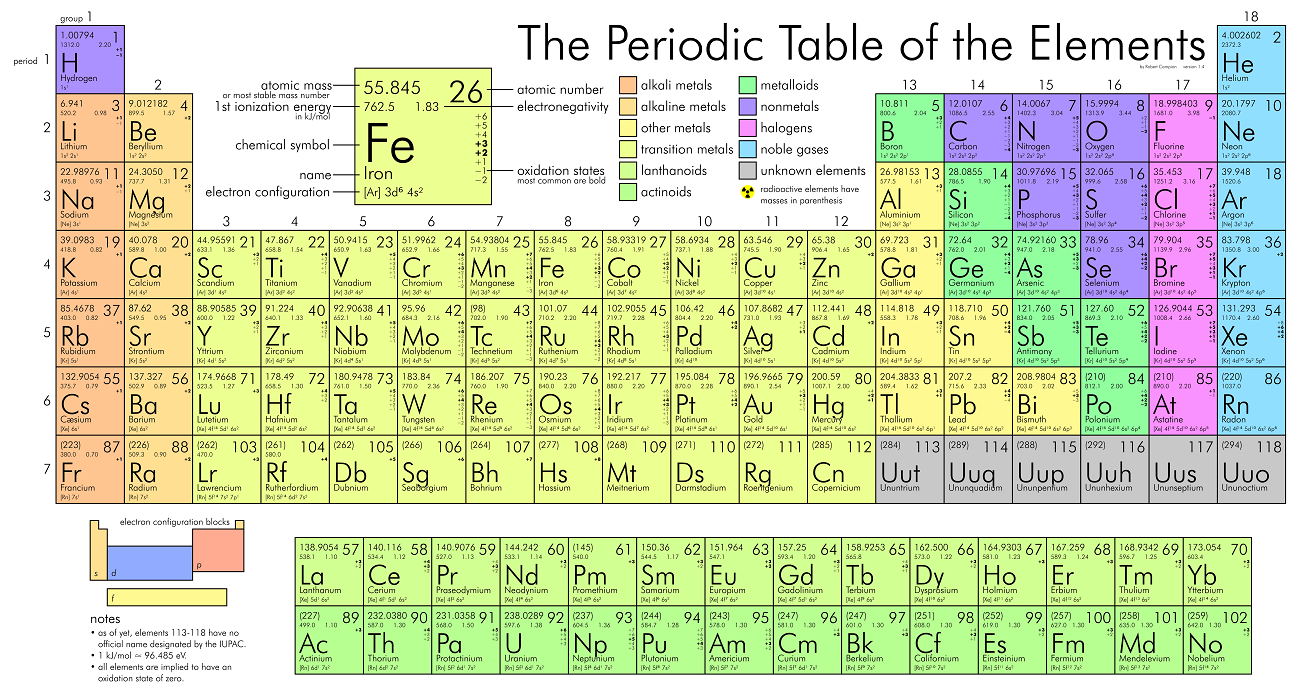
Trends On The Periodic Table
- The first column are the
Alkali metals.- They are shiny, have the consitency of clay, and are easily cut with a knife.
- They are the most reactive metals.
- They react violently with water.
- Alkali metals are never found as free elements in nature. They are always bonded with another element.
- The second column are the
Alkaline earth metals.- They are never found uncombined in nature.
- The last column are the
Noble gases.- Extremely un-reactive.
- The second last column are the
Halogens.- The most reactive non-metals
- They react with alkali metals to form salts.
- The middle parts are the
transition metals.- They are good conductors of heat and electricity.
- Usually bright coloured.
- They have properties similar to elements in their same family
- Many of them combine with oxygen to form compounds called oxides.
- The rows outside the table are the
Inner tranistion metals.

- The left to the staircase are the
metals and the right are the non-metals. The ones
touching the staircase are the
metalloids.

How To Read An Element

History of The Atom
| Person | Description | Picture |
|---|---|---|
| Democritus | All matter can be divided up into smaller
pieces until it reaches an unbreakable particle called an ATOM (cannot
be cut) He proposed atoms are of diffent sizes, in constant motion and separated by empty spaces |
|
| Aristole | - Rejected Democritus ideas, believed all matter was made up the 4 elements, it was accepted for nearly 2000 years |  |
| John Dalton | - Billbard model, atoms of
different elements are different Atoms are never created or destroyed. - Atoms of an element are identical |
|
| JJ Thomson | - Atoms contain negatively charged
electrons, since atoms are neutral, the rest of the atom is a
positevly charged sphere. - Negatively charged electrons were evenly distrubuted throughout the atom. - Ray cathode experiment - basically atoms were attracted to a postive end of the tube, so there most be negative charges in the atoms.  |
 |
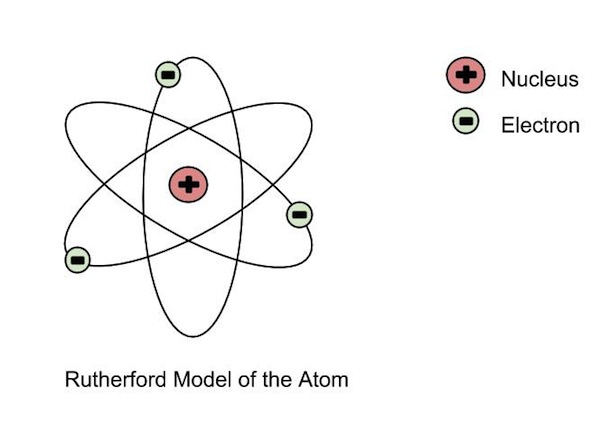
| Ernest Rutherford | - Discovered that the postively charged
nucleus. - The nucleus was surrounded by a cloud of negatively charged electrons - Most of the atom was just space. - Gold foil experiement, alpha particles (postively charged) shot at atom, some bounced off at weird angles, so there most be a postively charged thing there. 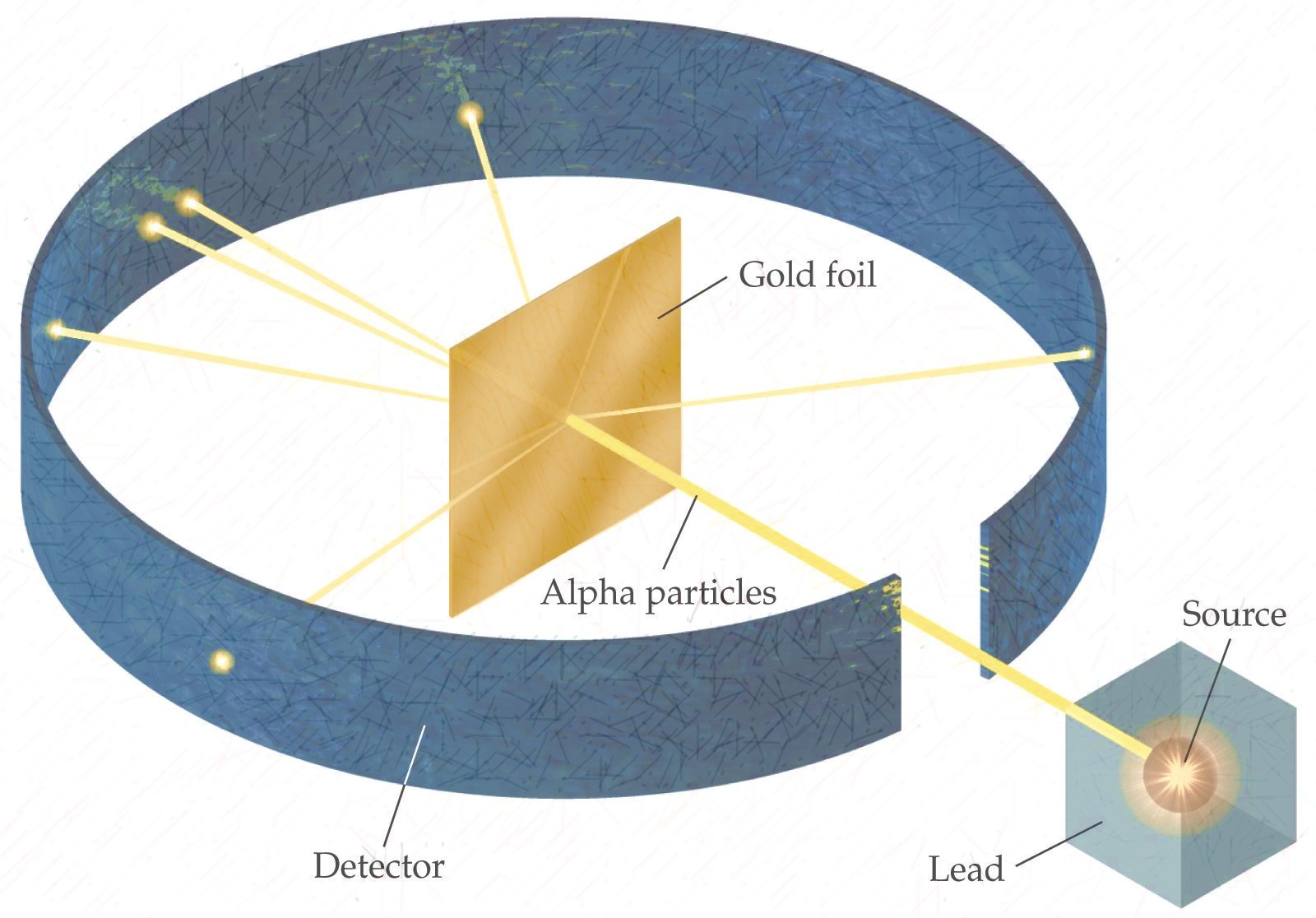 |
 |
| Niels Bohr | - Discovered that electrons orbit
the nucleus in fixed paths, each electron has a
definite amount of energy, further from nucles = more
energy. - Electrons cannot jump orbit to orbit or release energy as light going down. - Each orbit can hold a specifc amount of electrons, 2,8,8,2, useful for the first 20
elements |
 |
| James Chadwick | - Discovered the neutron, mass of neutron
= mass of proton (basically) - Neutral atoms have equal numbers of protons and electrons. |
 |
Carbon
Atoms
- Subscripts - tells us how many of the atom are there, for example N2 means there are 2 nitrongen atoms.
- Use distrubutive property if there are brackets and a subscript, for example, (CO)2 is equilivant to C2O2.
- Atoms are stable if they have a full valence shell (noble gases)
- Each family has the same amount of valence electrons as their family
number, so
alkali metalswould have 1 valence electron,alkaline earth metalswill have 2,halogens will have7 andnoble gaseswould have 8. - They will also have the same amount of protons as their
atomic number. - Number of protons = Number of electrons.
- Number of neutrons = mass - atomic number/number of protons.
Bohr-Rutherford / Lewis-Dot Diagrams
- Bohr-Rutherford
- Draw nucleus, and draw the apprioate number of orbits.
- Put number of protons and neutrons in the nucleus.
- Draw the correct number of electrons in each orbit

- Lewis-Dot Diagrams
- Draw element symbol
- Put the right number of valence electrons around the symbol,
perferably in pairs

Bonding
- To combine 2 atoms, each element wants to be stable. So they each want a full valence shell, (outer shell) so they are stable.
- They can either
gain,loseorshareelectrons in order to become stable. - Example:
- Oxygen and Hydrogen, in order to become stable, they all need 8
valence electrons. Hydrogen has 1, oxygen has 6, so we bring in another
hyrdogen and we let them share all their electrons, turning into
H2O, or water.
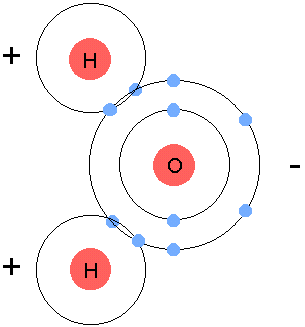
- Oxygen and Hydrogen, in order to become stable, they all need 8
valence electrons. Hydrogen has 1, oxygen has 6, so we bring in another
hyrdogen and we let them share all their electrons, turning into
H2O, or water.
- Use arrows to show gaining or losing electrons.
- Circle to show sharing of electrons.
Naming of Ionic Bonds
- Write cation (metal) first
- Write anion (non-metal) second
- Change the ending of the non-metal to
ide.


Decomposition
- A chemical change used to break compounds down into simpler substances
- Energy must be ADDED
- Using electricity
- Adding thermal energy
Catalyst
- Substance that accelerates a chemical change without being consumed OR changed itself
Uses of Hydrogen Peroxide
- On cuts/scraps
- Blood has a catalyst = see bubbling O2
- Cleans contact lenses
- Bubbling removes dirt
- Bleaches
- React with compounds that provide color
- RESULT = no colour (bleach blond hair/teeth)
Unit 3: Biology
The Spheres of Earth
Atmosphere
- The layer of
gasesabove Earth’s surface, extending upward for hundreds of kilometers.78% nitrogen gas.21% oxygen gas.< 1% argon, water vapour, carbon dioxide & other gases.
- Critical to (almost all) life on Earth.
- Acts like a blanket & moderates surface temperature.
- Insulation prevents excessive heating during the day & excessive cooling during the night.
- Average surface temperature droup from 15C to -18C.
- Blocks some solar radiation (most ultraviolet light).
Biosphere
- The regions of Earth where
living organismsexist. - Describes the locations in which life can exist within the lithosphere, atmosphere and hydrosphere.
- Biosphere is thin in comparison to diameter of the Earth.
- ALL conditions required for life must be met and maintained within this thin layer of ground, water, and nutrients to survive.
Hydrosphere
- All the
waterfound on Earth, above and below the Earth’s surface. - Includes
- Oceans
- Lakes
- Ice
- Ground Water
- Clouds
- 97% of water on Earth is in the oceans.
Lithosphere
- The
hard partof Earth’s surface. - Rocky outer shell of Earth.
- Consists of:
- Rocks and minerals that make up mountains, ocean floors, and Earth’s solid landscape -Thickness: 50 - 150km.
Terms
Biotic: Living components (their remains AND features)- Bears, insects, micro-organisms, nests
Abiotic: Non-living components- Physical/chemical components
- Temperature, wind, humidity, precipitation, minerals, air pressure
Sustainability: The ability to maintain natural ecological conditions without interruption, weakening, or loss of value.Community- Individual from all of the DIFFERENT populations (communities of different species)
Ecosystem- Term given to the community and its interactions with the abiotic environment
Sustainable Ecosystem- An ecosystem that is maintained through natural processes
Ecological niche:- Every species interacts with other species and with its environment in a unique way. This is its role in an ecosystem (e.g. what it eats, what eats it, how it behaves, etc.)
Biodiversity: The variety of life in a particular ecosystem, also known as biological diversity.- Canada is home to about 140 000 to 200 000 species of plants and animals. Only 71 000 have been identified.
Species Richness: the number of species in an area.- Diverse ecosystem = high species richness.
- Higher close to the equator.
- Ex. Amazon rainforest home to more than 200 species of hummingbirds, Ontario only has a single species.
Population: A group of organisms of one species that interbreed and live in the same place and time.- Population often change due to both natural and artifical factors (human activity).
Carry Capcity: The maximum population size of a particular species that a given ecosystem can sustain.Pollution: harmful comtaminants released into the enviornment.Bioremediation: the use of micro-organisms to consume and break down environmental pollutants.Photosynthesis: The process in which the Sun’s energy (LIGHT) is converted (put together with) into chemical energy AS GLUCOSE (sugar).Succession: The gradual and usually predictable changes in the composition of a community and the abiotic condtions following a disturbance.Producer: Organism that makes its own energy-rich food using the Sun’s energy.Consumer: Organism that obtains its energy from consuming other organisms.Eutrophication: Overfertilzation of staganat bodies of water with nutrients
Types of Energy
Radiant Energy
- Energy that travels through EMPTY SPACE
Thermal Energy
- Form of energy TRANSFERED DURING HEATING/COOLING
- Keeps the Earth’s surface warm
- CANNOT provide organisms with energy to grow & function
Light Energy
- VISIBLE forms of radiant energy
- Can be used by some organisms (CANNOT be stored)
Chemical Energy
- Used by living organisms to perform functions (growth, reproduction, etc.)
- MUST be replaced as it is used
It starts with the sun …
- Energy radiates from the sun (UV)
- Earth is hit with the UV or light energy
- 70% of radiant energy is absorbed by
Hydrosphere&Lithosphere - Converted into thermal energy
- Warms the atmosphere, evaporates water & produces winds
- What happens with the rest?
- Approx.
30%is reflected back into space 0.023%absorbed by living organisms through photosynthesis
Why is Photosynthesis important?
Animals cannot make their own food (glucose, energy)
- Must get our food from plants.
Plants are the first step in the food chain
Oxygen released during photosynthesis is necessary for all living things
Cellular Respiration
- Process of converting sugar into carbon dioxide, water and energy
- Makes stored energy available for use
- Takes place in the mitochondria
- Original energy stored in the sugar is released
- Occurs continuously
- Does NOT require light energy
BOTH producers AND consumers perform cellular respiration
ALL humans are consumers (unless you’re the hulk)
Steps in Cellular Respiration
- Mitochondria takes in nutrients
- Glucose and Oxygen
- Breaks both nutrients down
- Creates energy for the cell
REVERSE of Photosynthesis
- Sugar breaks down into CARBON DIOXIDE and
WATER
- Release of energy when this happens
- Sugar breaks down into CARBON DIOXIDE and
WATER
Feeding Relationship
- Energy flow through an ecosystem in one direction, from the sun or inorganic compounds to autotrophs (producers) and then to various hetrotrophs (consumers).
- Food are a series of steps in which organisms transfers energy by eating or eaten (pg. 43).
- Food webs show the complex interactions within an ecosystem (pg. 44).
- Each step in a food chain or web is called a
trophiclevel. Producers make up the first step, consumers make up the higher levels. E.g. first trophic level are producers, second trophic level are primary consumers, etc.
ECOLOGICAL PYRAMIDS
- Food chains and food webs do not give any information about the numbers of organisms involved.
- This information can be shown through ecological pyramids.
- An ecological pyramid is a diagram that shows the amount of energy or matter contained within each trophic level in a food web or food chain.
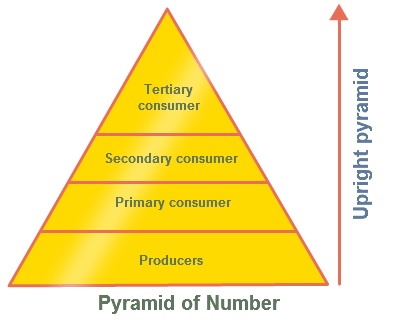
| Pyramid | Description | Picture |
|---|---|---|
| Pyramid of Biomass | Show the total amout of
living tissue available at each trophic level.
This shows the amount of tissue available for the next
trophic level. Biomass is preferred to the use of numbers of organisms because individual organisms can vary in size. It is the total mass (not the size) that is
important. Sometimes it’s inverted. Pyramid of biomass records the total dry organic matter of organisms at each trophic level in a given area of an ecosystem. |
 |
| Numbers Pyramids | Shows the number of organisms at each
trophic level per unit area of an ecosystem. Because each trophic level harvests only about one tenth of the energy
from the level below, it can support only about one 10th
the amount of living tissue. Can be inverted: 1 large tree supports
thousands of organisms living on it Pyramid of numbers displays the number of individuals |
 |
| Energy Pyramid | Shows the amount of energy input to each
trophic level in a given area of an ecosystem over an extended
period. CANNOT be inverted, due to energy transfers Only 10% of the energy available within one trophic level is transferred to organisms at the next trophic level |
 |
NOTE FOR ENERGY PYRAMIDS: In nature, ecological
efficiency varies from 5% to 20% energy
available between successive trophic levels (95% to
80% loss). About 10% efficiency is a general rule.
Rule of 10’s at each level.
Cycles
| Cycle | Picture |
|---|---|
| Water Cycle |  |
| Carbon Cycle |  |
| Nitrogen Cycle |  |
Water Cycle
- Continuous movement of water on, above and below the surface of the Earth ### Key Terms:
- Water moves from one reservoir to another (ocean to atmosphere,
river to lake)
- Evaporation, Condensation, Precipitation, Percolation (Infiltration), Run-off
- Forms: Solid (ice), Liquid (water), Gas (vapour)
STEPS/PROCESS:
- Exchange of energy leads to:
- Temperature Change, Climate
- Condenses 🡪 occurs during cooler temp
- Evaporation 🡪 happens during warmer temp
- Evaporation:
- purifies the water
- New fresh water for the land
- Flow of liquid water and ice
- Transports minerals across the globe
- Reshaping the geological features of Earth
- Erosion and sedimentation
Human Inpacts
- Humans building dams (flooding is a problem!)
- Deforestation contributes to global warming, hence melting glaciers and causing flooding in cities
- (Also less transpiration from clear cutting) – pg. 48
- Factories and cars pollute the air, leading to acid precipitation
- Oil spills destroy aquatic ecosystems
Carbon Cycle
- Fourth most abundant element in universe
- Building block of all living things
- Main Pathway – in and out of living matter
STEPS/PROCESSES
- All living organisms contain carbon
- CO2 is a waste product of cellular respiration
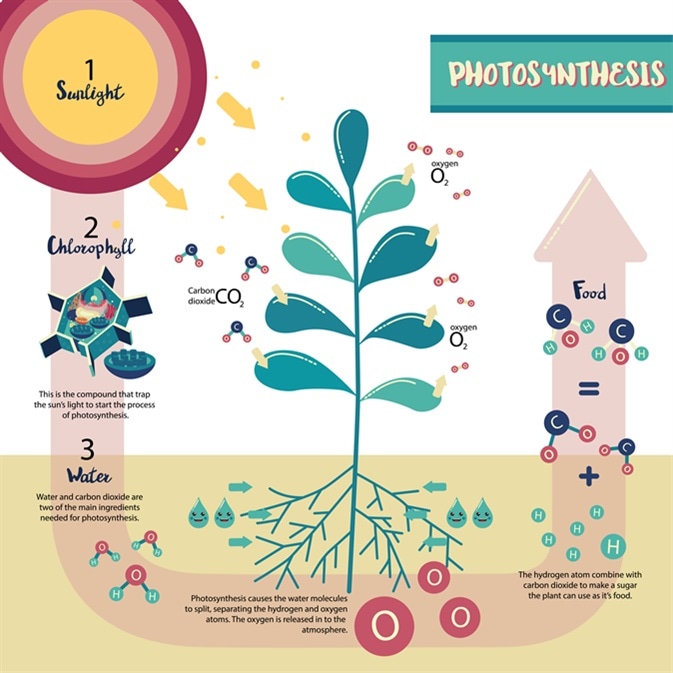
- Plants use carbon dioxide and water to form simple sugars (photosynthesis)
- Light Energy –> Chemical Energy
- Carbon dioxide is
returned to the enviornment by: 1.Resipirationin plants, animals & micro-organisms. 2.Decaycaused by micro-organisms (decompoers). 3.Combustion` i.e. Burning fossil fuels. - Phtosynthesis
- CO2 is converted to glucose using water and sunlight
- Cellular Respiration
- Breaks down glucose to release energy, expel CO2
- Oceans are a HUGE carbon sink.
Human Impacts
- Mining & burning fossil fuels: Speed up release of CO2 to the atmosphere.
- Deforestation & clearing vegetation: ↑ CO2 in atmosphere.
- Acid rain: release CO2 from limestone.
- CO2 in the atmosphere is now higher than it has been in at least 800 000 years.
Nitrogen Cycle
- The most abudant gas in the atmopshere (~78%)
Nitrogen Fixation: The process that causes the strong two-atom nitrogen molecules found in the atmopshere to break apart so they can combine with other atoms.Nitrogen gets fixed: Whenit is combined with oxygen or hydrogen.- An essential component of DNA, RNA, and protenis - the building blocks of life.
- Atmopspheric nitrogen = N2
- Most living organisms are
unableto use this form of nitrogen - Therefore, must be converted to a usable form!
- Most living organisms are
STEPS/PROCESSES
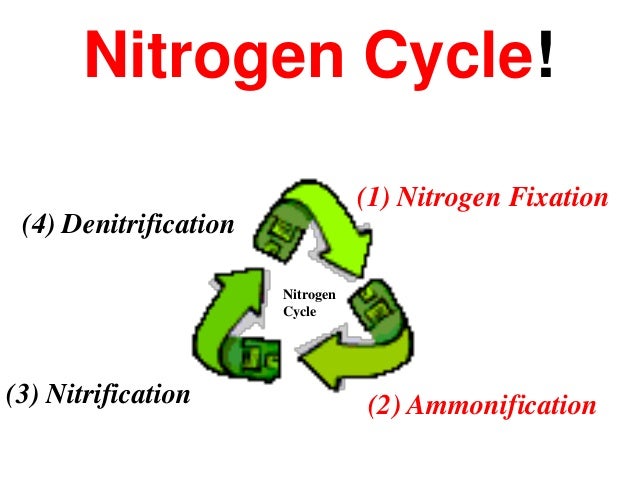
Nitrogen Fixation
- Most of the nitrogen used by living things is taken from the
atmosphere by certain bacteria in a process called
nitrogen fixation. - These microorganisms convert nitrogen gas into a variety of nitrogen containing compounds such as nitrates, nitrites, and ammonia
- Lightning and UV radiation also fix small amounts of it
- Humans add nitrogen to soil through fertilizer
- 3 ways nitrogen to get fixed
- Atmopheric Fixation
- Lightning Storms
- stroms and fuel burning in car engines produce nitrates, which are washed by rain into soil water.
- Lightning Storms
- Industrial Fixation
- Biological Fixation
- 2 types
- Free living Bacteria
- Highly specialized bacteria live in the soil and have the ability to combine atmospheric nitrogen with hydrogen to make ammonium(NH4+).
- Nitrogen changes into ammonium.
- Symbiotic Relationship Bacteria
- Bacteria live in the roots of legume family plants and provide the plants with ammonium(NH4+) in exchange for the plant’s carbon and a protected biome.
- Free living Bacteria
- 2 types
- Atmopheric Fixation
Nitritesare absorbed by plant roots and converted to plant protein.Nitratescan be absorbed by other plants to continue the cycle.Denitrifying bacteriaconvert soil nitrates into N2 gas- This is a
lossof N2 from the cycle
- This is a
Human Impacts
- Nitrates also
entersthe cycle through the addition of nitrogen rich fertilizers to the soil – made industrially from nitrogen gas (Eutrophication – pg. 60) - Factories release NO compounds (acid rain)
Nutrient Recycling
- There is a
limitedamount ofnutrientson earth- e.g. you are probably aware of the water cycle – where water is
constantly being
recycledin nature. - There are similar cycles for all nutrients.
- e.g. you are probably aware of the water cycle – where water is
constantly being
- When plants and animals die, their nutrient content is
not wasted. - Bacteria and fungi decompose the remains and release the nutrients back into the abiotic environment (i.e. into the soil, nearby water and air).
- Nutrients are then taken up by other plants and used to make new organic material.
- This material is passed on down the food chains and is reused by all the chain members.
- When death occurs for these members, the nutrients are again returned to the abiotic environment and the cycling of nutrients continues in this circular way.
- This ensures that there is no real longterm drain on the Earth’s nutrients, despite millions of years of plant and animal activity.
Changes In Population
- The carry capcacity of an ecosystem depends on numerous biotic and abiotic factors.
- These can be classified into two categories.
Density dependent factorsDensity independent factors
Density Independent Factors
- DIF’s can affect a population no matter what its density is. The effect of the factor (such as weather) on the size of the population does not depend on the original size of the population.
- Examples:
- unusual weather
- natural disasters
- seasonal cycles
- certain human activities—such as damming rivers and clear-cutting forests
Density Dependent Factors
- DDF’s affect a population ONLY when it reaches a certain size. The effect of the factor (such as disease) on the size of the population depends on the original size of the population
- Examples:
- Competition
- Predation
- Parasitism
- Disease
Relationships
- Symbiosis
- Two different organisms associate with each other in a close way.
- Is the interaction between members of
two different speciesthat live together in a close association. - Types
- Mutualism (+/+)
- Both species benefit from the relationship.
- (eg. human intestine and good bacteria, bees and flowers, clownfish and sea anemone, cattle egret and cow).
- Commensalism (+/0)
- one species benefits, the other is unaffected.
- (eg. beaver cutting down trees, whales and barancles).
- Parasitism (-/+)
- one species is harmed, the other benefits.
- (eg. lice and humans, mosquito and humans).
- Competition (-/-)
- neither species benefits. Can be harmed. (-/-).
- Neutralism (0/0)
- both species are unaffected (unlikely).
- True neutralism is extremely unlikely or even impossible to prove. One cannot assert positively that there is absolutely no competition between or benefit to either species.
- Example: fish and dandelion
+ Parasitism and Predation Commensalism Mutalism 0 Neutralism Commensalism - Competition Parasitism and Predation - 0 + - Mutualism (+/+)
- Competition
- Individuals compete for limited resources
- Types
- Intraspecific Competition
- Is the competition between individuals of the same species.
- (eg. male deer uses antlers to fight each other for mates, little herons compete for food).
- Interspecific Competition
- Is the competition between individuals of different species.
- (eg. cardinals and blue jays at a bird feeder, lions and hyenas competing for food).
- Intraspecific Competition
- Types
- Individuals compete for limited resources
- Predation
- One animal eats (kills) another
Reasons To Compete
- Food and water.
- Space (habitat).
- Mates.
Candian Biomes

Ecosystem Services
- Cultural Services
- Benefits relating to our enjoyment of the environment.
- Ex. Recreational, aesthetic and spiritual experiences when we interact with natural surroundings.
- Ecotourism: tourists engage in environmentally responsible travel to relatively undisturbed natural areas.
- Ex. Canada’s Wilderness.
- Ecosystem Products
- Humans use products produced by the ecosystem.
- Hunt animals and harvest plants, lakes/oceans supply us with seafood.
- Terrestrial: ecosystems: medicines, fibres, rubber and dyes.
- Forestry: largest industries and employers.
- Regulate and maintain important abiotic and biotic features of
environment.
- Cycle water, oxygen, and nutrients.
- Help protect us from physical threats.
- Plant communities protect the soil from wind and water erosion.
- Ecosystems act as sponges.
- Absorb water and slowly release it into the groundwater and surface water (reduces erosion and protects against flooding, filters the water).
- Protect land from storms along coasts where wave damage erodes the
shoreline.
- Mangroves
Monetary Value of Ecosystem Services
- Very difficult to put a dollar value to it.
- Dollar value of cleaning the air/water, moderating climate and providing paper fibre, medicines and other products is HIGH.
- Ranges into the trillions of dollars/year (maybe 60 trillion?).
- Provides valuable services that are free and renewable.
Successions
- Natural ecosystems are in a state of equilibrium (their biotic and abiotic features remain relatively constant over time).
- Equilibrium is established when abiotic conditions are stable.
- Photosynthesis and cellular respiration are balanced.
- Populations are healthy and stable.
- Small ecosystems are in a constant state of change.
- Forest fire or disease outbreak can cause short-term changes on a local level.
- Types
Primary
- on newly epxposed ground, such asa following a volcanic eruption.
Secondary
- in a partially distrubed ecosystem, such as following a forest fire.
- Human caused disturbances.
- Results in gradual changes as plants, animals, fungi and micro organisms become established in an area.
- In both terrestrial and aquatic ecosystems.
Benefits of Succession
- Provides a mechanism by which ecosystems maintain their long term sustainability.
- Allows ecosystems to recover from natural or human caused disturbances.
- Offers hope (New Orleans, New Jersey, Florida, Puerto Rico).
- Time needed is very long.
- Original cause of disturbance must be eliminated.
- Not all disturbances can be repaired.
- Disturbances can be repaired through human actions that support the natural processes of succession.
Species at Risk
- Do not have to be driven to extinction for there to be ecological consequences.
- Population falls below critical level = ecological niche can no longer be filled.
- Consequences for abiotic and biotic features.
- Extirpated: no longer exists in a specific area.
- Endangered: facing imminent extirpation or extinction.
- Threatened: likely to become endangered if factors reducing its survival are not changed.
- Special Concern: may become threatened or endangered because of a combination of factors.