mirror of
https://gitlab.com/magicalsoup/Highschool.git
synced 2025-01-23 16:11:46 -05:00
15 KiB
15 KiB
Unit 2: Chemistry
Chemistry Vocabulary List
| Word | Definition (or diagram/translation) |
|---|---|
| Particle Theory of Matter | Theory that describes the composition and behaviour of matter as being composed of small particles with empty space |
| Matter | Substance that has mass and occupies space |
| Mechanical Mixture | A heterogeneous mixture which one can physically separate |
| Suspension | A heterogeneous mixture where insoluble solid particles are distributed throughout a fluid, floating freely/td> |
| Alloy | A combination of 2+ metals |
| Mixture | A substance that is made up of at least 2 types of particles |
| Qualitative property | A property of a substance that is not measured and doesn’t have a numerical value, such as colour, odour, and texture |
| Qualitative observation | An numerical observation |
| Precipitate | A solid that separates from a solution |
| Density | A measure of how much mass is contained in a given unit volume of a substance; calculated by dividing the mass of a sample of its volume (mass/volume) |
| Element | Element An element is made up of the same atoms throughout, and cannot be broken down further |
| Metal | a solid material that is typically hard, shiny, malleable, fusible, and ductile, with good electrical and thermal conductivity |
| Pure substance | A substance that is made up of only one type of particle |
| Atom | The smallest unit of matter found in substances |
| Solution | A uniform mixture of 2 or more substances |
| Colloid | is substance with small particles suspended in it, unable to be separated by gravity |
| Emulsion | A mixture of 2 insoluble liquids, in which one liquid is suspended in the other |
| Physical Property | Characteristic of a substance that can be determined without changing the makeup of the substance |
| Characteristic | A physical property that is unique to a substance and can be used to identify the substance |
| Periodic Table | a table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure (and hence similar chemical properties) appear in vertical columns. |
| Compound | Compounds are chemically joined atoms of different elements |
| Non-Metal | A substance that isn’t a metal |
| Physical Change |
A change in which the composition of the substance remains
unalteredandno new substances are produced
|
| Chemical Change |
A change in the starting substance and the production of ONE
or more new substances Original substance does not disappear BUT the composition is rearranged |
| Molecule | Two or more non-metal atoms joined together |
| Diatomic Molecules |
Molecules that only consists of 2 elements H O F BR I N C L - hyrodgen, oxygen, fluorine, bromine, iodine, nitrogen, chlorine. |
| Ions | A Charged particle, that results from a loss (cation - positve, less electrons) or gain (anion - negative, more electrons) of electrons when bonding |
| Electron | Negatively Charged |
| Proton | Positively Charged |
| Neutron | Neutral Charged |
| Ionic Charge | The sum of the positive and negative charges in a ion |
| Covalent Bond | The sharing of electrons between atoms when bonding |
| Valence Electrons | Number of electrons on the most outer orbit/shell of the element |
Particle Theory of Matter
- Matter is made up of tiny particles.
- Particles of Matter are in constant motion.
- Particles of Matter are held together by very strong electrical forces.
- There are empty spaces between the particles of matter that are very large compared to the particles themselves.
- Each substance has unique particles that are different from the particles of other substances.
Physical Properties
- A characeristic of a substance that can be determined without changing the composition (“make-up”) of that substance
- Characteristics can be determinded using your 5 senses and measuring
instruments
- smell, taste, touch, hearing, sight
- scales, tape, measuring meter
The 3 States Of Matter
| Solid | Liquid | Gas |
|---|---|---|
 |
 |
 |
| - Holds Shape - Fixed Volume |
- Shape of Container - Free Surface - Fixed Volume |
- Shape of Container - Volume of Container |
Qualitative and Quantitative Properties
| Type | Definition | Example |
|---|---|---|
| Quantitative Property | A property that IS measured and has
a numerical value |
Ex.
Temperature, height, mass, density |
| Qualitative Property | A property that is NOT measured and has
no numerical value |
Ex.
Colour, odor, texture |
Density

Quantitative physical Properties
Density: amount ofstuff(or mass) per unit volume (g/cm3)Freezing Point: point where water solidifies (0oC)Melting Point: point where water liquefies (0oC)Boiling Point: point where liquid phase becomes gaseous (100oC)
Common Qualitative Physical Properties
| Type | Definition | Example |
|---|---|---|
| Lustre | Shininess of dullness Referred to as high or low lustre depending on the shininess |
|
| Clarity | The ability to allow light through | Transparent (Glass)
Translucent (Frosted Glass) Opaque
(Brick) |
| Brittleness | Breakability or flexibility Glass would be considered as brittle whereas slime/clay are flexible |
|
| Viscosity | The ability of a liquid or gas to resist
flow or not pour readily through Refer to as more or less viscous |
Molasses is more viscous, water is less (gases tend to get”thicker as heated; liquids get runnier) |
| Hardness | The relative ability to scratch or be
scratched by another substance Referred to as high or low level of hardness |
Can use a scale (1 is wax, 10 is diamond) |
| Malleability | the ability of a substance
to be hammered into a thinner sheet or molded |
Silver is malleable Play dough/pizza dough is less glass is not malleable |
| Ductility | the ability of a substance to be pulled into a finer strand | Pieces of copper can be drawn into thin wires, ductile |
| Electrical Conductivity | The ability of a substance to allow
electric current to pass through it Refer to as high and low conductivity |
Copper wires have high conductivity Plastic has no conductivity |
| Form: Crystalline Solid | Have their particles arranged in an orderly geometric pattern | Salt and Diamonods |
| Form: Amorphous Solid | Have their particles randomly distributed without any long-range-pattern | Plastic, Glass, Charcoal |
Chemical Property
- A characteristic (property) of a substance that describes its
ability to undergo
changes to its composition to produce one of more new substances. AKA BEHAVIOUR. Everything has one! Cannot be determined by physical properties- E.g. ability of nails /cars to rust
- Fireworks are explosive
- Denim is resistant to soap, but is combustible
- Baking soda reacts with vinegar and cake ingredients to rise
- Bacterial cultures convert milk to cheese, grapes to wine, cocoa to chocolate
- CLR used to clean kettles, showerheads because it breaks down minerals
- Silver cleaner for tarnished jewellery, dishes because silver reacts with air to turn black.
Periodic Table
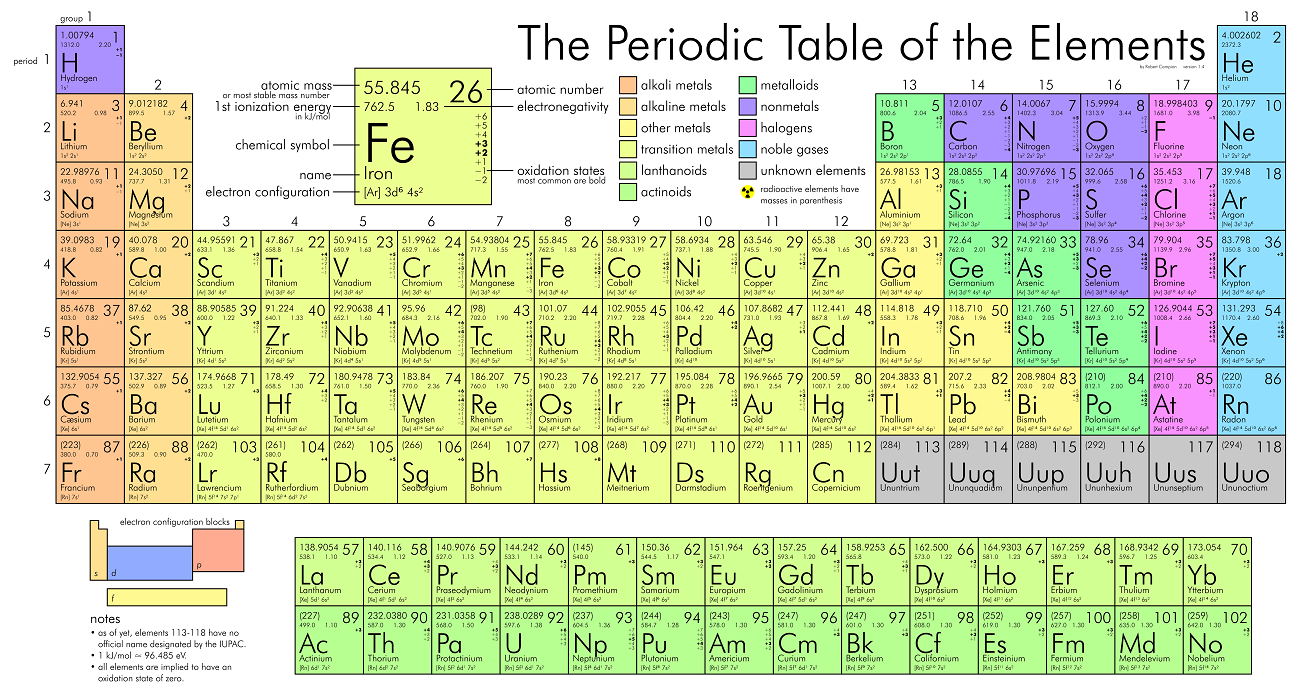
Trends On The Periodic Table
- The first column are the
Alkali metals.- They are shiny, have the consitency of clay, and are easily cut with a knife.
- They are the most reactive metals.
- They react violently with water.
- Alkali metals are never found as free elements in nature. They are always bonded with another element.
- The second column are the
Alkaline earth metals.- They are never found uncombined in nature.
- The last column are the
Noble gases.- Extremely un-reactive.
- The second last column are the
Halogens.- The most reactive non-metals
- They react with alkali metals to form salts.
- The middle parts are the
transition metals.- They are good conductors of heat and electricity.
- Usually bright coloured.
- They have properties similar to elements in their same family
- Many of them combine with oxygen to form compounds called oxides.
- The rows outside the table are the
Inner tranistion metals.

- The left to the staircase are the
metals and the right are the non-metals. The ones
touching the staircase are the
metalloids.

How To Read An Element

History of The Atom
| Person | Description | Picture |
|---|---|---|
| Democritus | All matter can be divided up into smaller
pieces until it reaches an unbreakable particle called an ATOM (cannot
be cut) He proposed atoms are of diffent sizes, in constant motion and separated by empty spaces |
|
| Aristole | - Rejected Democritus ideas, believed all matter was made up the 4 elements, it was accepted for nearly 2000 years |  |
| John Dalton | - Billbard model, atoms of
different elements are different Atoms are never created or destroyed. - Atoms of an element are identical |
|
| JJ Thomson | - Atoms contain negatively charged
electrons, since atoms are neutral, the rest of the atom is a
positevly charged sphere. - Negatively charged electrons were evenly distrubuted throughout the atom. - Ray cathode experiment - basically atoms were attracted to a postive end of the tube, so there most be negative charges in the atoms.  |
 |
| Ernest Rutherford | - Discovered that the postively charged
nucleus. - The nucleus was surrounded by a cloud of negatively charged electrons - Most of the atom was just space. - Gold foil experiement, alpha particles (postively charged) shot at atom, some bounced off at weird angles, so there most be a postively charged thing there.  |
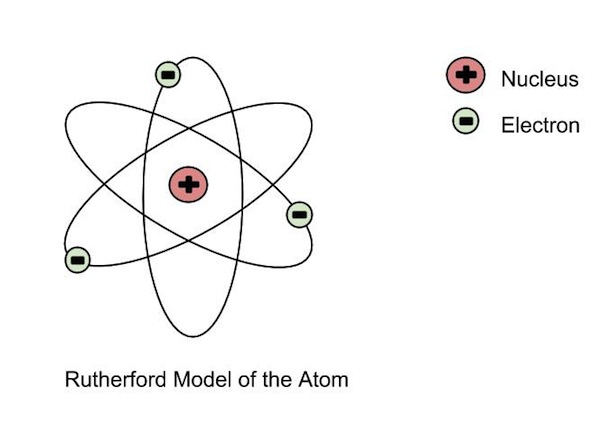 |
| Niels Bohr | - Discovered that electrons orbit
the nucleus in fixed paths, each electron has a
definite amount of energy, further from nucles = more
energy. - Electrons cannot jump orbit to orbit or release energy as light going down. - Each orbit can hold a specifc amount of electrons, 2,8,8,2, useful for the first 20
elements |
 |
| James Chadwick | - Discovered the neutron, mass of neutron
= mass of proton (basically) - Neutral atoms have equal numbers of protons and electrons. |
 |
Carbon
Atoms
- Subscripts - tells us how many of the atom are there, for example N2 means there are 2 nitrongen atoms.
- Use distrubutive property if there are brackets and a subscript, for example, (CO)2 is equilivant to C2O2.
- Atoms are stable if they have a full valence shell (noble gases)
- Each family has the same amount of valence electrons as their family
number, so
alkali metalswould have 1 valence electron,alkaline earth metalswill have 2,halogens will have7 andnoble gaseswould have 8. - They will also have the same amount of protons as their
atomic number. - Number of protons = Number of electrons.
- Number of neutrons = mass - atomic number/number of protons.
Bohr-Rutherford / Lewis-Dot Diagrams
- Bohr-Rutherford
- Draw nucleus, and draw the apprioate number of orbits.
- Put number of protons and neutrons in the nucleus.
- Draw the correct number of electrons in each orbit

- Lewis-Dot Diagrams
- Draw element symbol
- Put the right number of valence electrons around the symbol,
perferably in pairs

Bonding
- To combine 2 atoms, each element wants to be stable. So they each want a full valence shell, (outer shell) so they are stable.
- They can either
gain,loseorshareelectrons in order to become stable. - Example:
- Oxygen and Hydrogen, in order to become stable, they all need 8
valence electrons. Hydrogen has 1, oxygen has 6, so we bring in another
hyrdogen and we let them share all their electrons, turning into
H2O, or water.
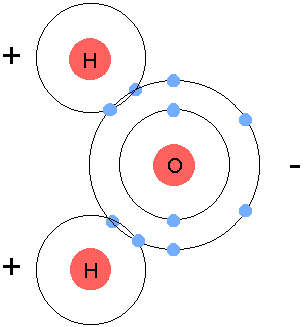
- Oxygen and Hydrogen, in order to become stable, they all need 8
valence electrons. Hydrogen has 1, oxygen has 6, so we bring in another
hyrdogen and we let them share all their electrons, turning into
H2O, or water.
- Use arrows to show gaining or losing electrons.
- Circle to show sharing of electrons.
Naming of Ionic Bonds
- Write cation (metal) first
- Write anion (non-metal) second
- Change the ending of the non-metal to
ide.


Decomposition
- A chemical change used to break compounds down into simpler substances
- Energy must be ADDED
- Using electricity
- Adding thermal energy
Catalyst
- Substance that accelerates a chemical change without being consumed OR changed itself
Uses of Hydrogen Peroxide
- On cuts/scraps
- Blood has a catalyst = see bubbling O2
- Cleans contact lenses
- Bubbling removes dirt
- Bleaches
- React with compounds that provide color
- RESULT = no colour (bleach blond hair/teeth)